
A steam power plant operates on an ideal reheat–regenerative Rankine cycle with one reheater and two open feedwater heaters. Steam enters the high-pressure turbine at 1500 psia and 1100°F and leaves the low- pressure turbine at 1 psia. Steam is extracted from the turbine at 250 and 40 psia, and it is reheated to 1000°F at a pressure of 140 psia. Water leaves both feedwater heaters as a saturated liquid. Heat is transferred to the steam in the boiler at a rate of 4 × 105 Btu/s. Show the cycle on a T-s diagram with respect to saturation lines, and determine (a) the mass flow rate of steam through the boiler, (b) the net power output of the plant, and (c) the thermal efficiency of the cycle.
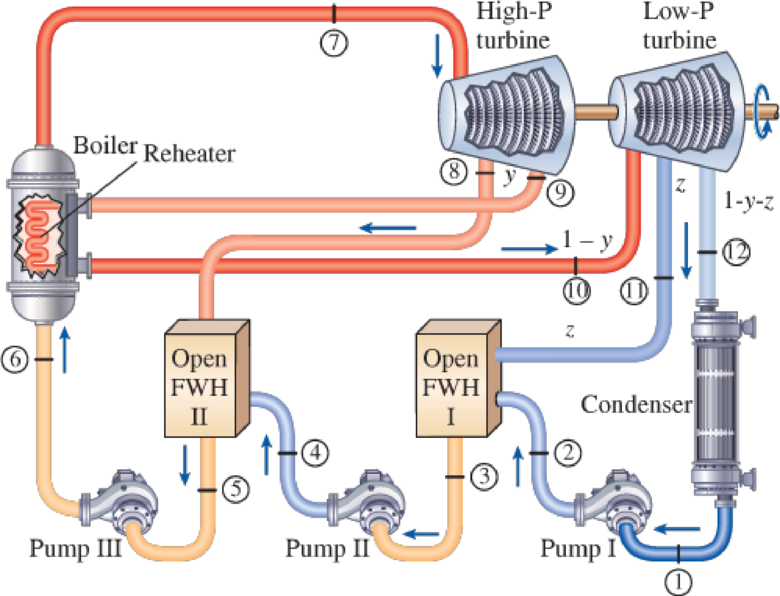
FIGURE P10–62E
(a)
The mass flow rate of steam flowing through the boiler.
Answer to Problem 62P
The mass flow rate of steam flowing through the boiler is
Explanation of Solution
Draw the schematic layout of the given power plant that operates on an ideal reheat-regenerative Rankine cycle as shown in Figure 1.
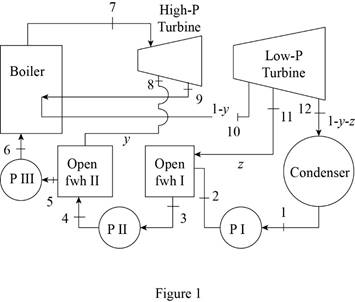
Draw the
Figure 2.
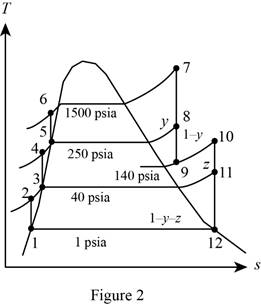
Here, water (steam) is the working fluid of the ideal regenerative Rankine cycle. The cycle involves three pumps.
Write the formula for work done by the pump during process 1-2.
Here, the specific volume is
Write the formula for enthalpy
Write the formula for work done by the pump during process 3-4.
Here, the specific volume is
Write the formula for enthalpy
Write the formula for work done by the pump during process 5-6.
Here, the specific volume is
Write the formula for enthalpy
Before reheating.
At state 9:
The steam expanded to the pressure of
After reheating.
At state 10:
The steam is reheated to the temperature of
At state 12:
The steam enters the condenser at the pressure of
The quality of water at state 12 is expressed as follows.
The enthalpy at state 12 is expressed as follows.
Here, the enthalpy is
Write the formula for heat in
Here, the mass fraction steam extracted from the turbine to the feed water entering the boiler via feed water heater-II
Write the general equation of energy balance equation.
Here, the rate of net energy inlet is
At steady state the rate of change of net energy of the system
Refer Equation (XI).
Write the energy balance equation for open feed water heater-II.
Rewrite the Equation (XII) in terms of mass fraction
Refer Equation (XI).
Write the energy balance equation for open feed water heater-I.
Rewrite the Equation (XIV) in terms of mass fraction
Write the formula for mass flow rate.
Here, the rate of heat input is
At state 1:
The water exits the condenser as a saturated liquid at the pressure of
Refer Table A-5E, “Saturated water-Pressure table”.
The enthalpy
At state 3: (Pump II inlet)
The water exits the open feed water heater-I as a saturated liquid at the pressure of
Refer Table A-5E, “Saturated water-Pressure table”.
The enthalpy
At state 5: (Pump III inlet)
The water exits the open feed water heater-II as a saturated liquid at the pressure of
Refer Table A-5E, “Saturated water-Pressure table”.
The enthalpy
At state 7: (H.P. Turbine inlet)
The steam enters the turbine as superheated vapor.
Refer Table A-6E, “Superheated water”.
The enthalpy
Refer Figure 2.
At state 8:
The steam is extracted at the pressure of
Refer Table A-6E, “Superheated water”.
The enthalpy
At state 9:
The steam is expanded at the pressure of
Refer Table A-6E, “Superheated water”.
The enthalpy
At state 10:
The steam is reheated to the temperature of
Refer Table A-6E, “Superheated water”.
The enthalpy
Refer Figure 2.
At state 11:
The steam is expanded at the pressure of
Refer Table A-6E, “Superheated water”.
The enthalpy
At state 12: (Condenser inlet)
The steam enters the condenser at the pressure of
Refer Table A-5E, “Saturated water-Pressure table”.
Obtain the following properties corresponding to the pressure of
Conclusion:
Substitute
Substitute
Substitute
Equation (III).
Substitute
Substitute
Equation (V).
Substitute
From Figure 2.
Substitute
Substitute
Equation (VIII).
Consider the open feed water heater-II alone.
Substitute
Equation (XIII).
Consider the open feed water heater-I alone.
Substitute
Substitute
Substitute
Substitute
Thus, the mass flow rate of steam flowing through the boiler is
(b)
The net power output of the plant.
Answer to Problem 62P
The net power output of the plant is
Explanation of Solution
Write the formula for net power output of the cycle per unit mass.
Write the formula for net power output of the cycle.
Here, the mass flow rate is
Conclusion:
Substitute
Substitute
Thus, the net power output of the plant is
(c)
The thermal efficiency of the cycle.
Answer to Problem 62P
The thermal efficiency of the cycle is
Explanation of Solution
Write the formula for thermal efficiency of the cycle
Conclusion:
Substitute
Thus, the thermal efficiency of the cycle is
Want to see more full solutions like this?
Chapter 10 Solutions
Thermodynamics: An Engineering Approach
Additional Engineering Textbook Solutions
Starting Out with Java: From Control Structures through Objects (7th Edition) (What's New in Computer Science)
Fluid Mechanics: Fundamentals and Applications
Java How to Program, Early Objects (11th Edition) (Deitel: How to Program)
Database Concepts (8th Edition)
Mechanics of Materials (10th Edition)
Starting Out With Visual Basic (8th Edition)
- Prob 2. The material distorts into the dashed position shown. Determine the average normal strains &x, Ey and the shear strain Yxy at A, and the average normal strain along line BE. 50 mm B 200 mm 15 mm 30 mm D ΕΙ 50 mm x A 150 mm Farrow_forwardProb 3. The triangular plate is fixed at its base, and its apex A is given a horizontal displacement of 5 mm. Determine the shear strain, Yxy, at A. Prob 4. The triangular plate is fixed at its base, and its apex A is given a horizontal displacement of 5 mm. Determine the average normal strain & along the x axis. Prob 5. The triangular plate is fixed at its base, and its apex A is given a horizontal displacement of 5 mm. Determine the average normal strain &x along the x' axis. x' 45° 800 mm 45° 45% 800 mm 5 mmarrow_forwardAn airplane lands on the straight runaway, originally travelling at 110 ft/s when s = 0. If it is subjected to the decelerations shown, determine the time t' needed to stop the plane and construct the s -t graph for the motion. draw a graph and show all work step by steparrow_forward
- dny dn-1y dn-1u dn-24 +a1 + + Any = bi +b₂- + +bnu. dtn dtn-1 dtn-1 dtn-2 a) Let be a root of the characteristic equation 1 sn+a1sn- + +an = : 0. Show that if u(t) = 0, the differential equation has the solution y(t) = e\t. b) Let к be a zero of the polynomial b(s) = b₁s-1+b2sn−2+ Show that if the input is u(t) equation that is identically zero. = .. +bn. ekt, then there is a solution to the differentialarrow_forwardB 60 ft WAB AB 30% : The crane's telescopic boom rotates with the angular velocity w = 0.06 rad/s and angular acceleration a = 0.07 rad/s². At the same instant, the boom is extending with a constant speed of 0.8 ft/s, measured relative to the boom. Determine the magnitude of the acceleration of point B at this instant.arrow_forwardThe motion of peg P is constrained by the lemniscate curved slot in OB and by the slotted arm OA. (Figure 1) If OA rotates counterclockwise with a constant angular velocity of 0 = 3 rad/s, determine the magnitude of the velocity of peg P at 0 = 30°. Express your answer to three significant figures and include the appropriate units. Determine the magnitude of the acceleration of peg P at 0 = 30°. Express your answer to three significant figures and include the appropriate units. 0 (4 cos 2 0)m² B Aarrow_forward
- 5: The structure shown was designed to support a30-kN load. It consists of a boom AB with a 30 x 50-mmrectangular cross section and a rod BC with a 20-mm-diametercircular cross section. The boom and the rod are connected bya pin at B and are supported by pins and brackets at A and C,respectively.1. Calculate the normal stress in boom AB and rod BC,indicate if in tension or compression.2. Calculate the shear stress of pins at A, B and C.3. Calculate the bearing stresses at A in member AB,and in the bracket.arrow_forward4: The boom AC is a 4-in. square steel tube with a wallthickness of 0.25 in. The boom is supported by the 0.5-in.-diameter pinat A, and the 0.375-in.-diameter cable BC. The working stresses are 25ksi for the cable, 18 ksi for the boom, and 13.6 ksi for shear in the pin.Neglect the weight of the boom.1. Calculate the maximum value of P (kips) based on boom compression and the maximum value of P (kips) based on tension in the cable.2. Calculate the maximum value of P (kips) based on shear in pin.arrow_forward3: A steel strut S serving as a brace for a boat hoist transmits a compressive force P = 54 kN to the deck of a pier as shown in Fig. STR-08. The strut has a hollow square cross section with a wall thickness t =12mm and the angle θ between the strut and the horizontal is 40°. A pin through the strut transmits the compressive force from the strut to two gusset plates G that are welded to the base plate B. Four anchor bolts fasten the base plate to the deck. The diameter of the pin is 20mm, the thickness of the gusset plates is 16mm, the thickness of the base plate is 8mm, and the diameter of the anchor bolts is 12mm. Disregard any friction between the base plate and the deck.1. Determine the shear stress in the pin, in MPa and the shear stress in the anchor bolts, in MPa.2. Determine the bearing stress in the strut holes, in MPa.arrow_forward
- 1. In the figure, the beam, W410x67, with 9 mm web thicknesssubjects the girder, W530x109 with 12 mm web thickness to a shear load,P (kN). 2L – 90 mm × 90 mm × 6 mm with bolts frame the beam to thegirder.Given: S1 = S2 = S5 = 40 mm; S3 = 75 mm; S4 = 110 mmAllowable Stresses are as follows:Bolt shear stress, Fv = 125 MPaBolt bearing stress, Fp = 510 MPa1. Determine the allowable load, P (kN), based on the shearcapacity of the 4 – 25 mm diameter bolts (4 – d1) and calculate the allowable load, P (kN), based on bolt bearing stress on the web of the beam.2. If P = 450 kN, determine the minimum diameter (mm) of 4 – d1based on allowable bolt shear stress and bearing stress of thebeam web.arrow_forward6: The 6-kN load P is supported by two wooden members of 75 x 125-mm uniform cross section that are joined by the simple glued scarf splice shown.1. Calculate the normal stress in the glue, in MPa.2. Calculate the shear stress in the glue, in MPa.arrow_forwardUsing Matlab calculate the following performance characteristics for a Tesla Model S undergoing the 4506 drive cycle test Prated Trated Ebat 80kW 254 Nm 85kWh/1645kg MUEH A rwheel 0.315M 133.3 C 0.491 Ng ng 7g 8.190.315 8.19 0.315 7ed= 85% Ebpt 35-956 DRIVE AXLE Ebfb chę =85% V Minverter H/A Battery Charger En AC Pry 9) required energy output from the motor to drive this cycle Cassume no regenerative braking) b) range of the Tesla Model S for this drive cycle (assume no regenerative breaking c) estimated mpge cycle of the Tesla Model S for this drive Cassume no regenerative breaking) d) Recalculate parts abc now assuming you can regenerate returns correctly due to inefficiency. from braking. Be careful to handle the diminishing energy braking makes in terms of required e) Quantify the percentage difference that regenerative required energy, range and mpge, DI L Ta a ra OLarrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





