
A steam power plant operates on an ideal regenerative Rankine cycle with two open feedwater heaters. Steam enters the turbine at 8 MPa and 550°C and exhausts to the condenser at 15 kPa. Steam is extracted from the turbine at 0.6 and 0.2 MPa. Water leaves both feedwater heaters as a saturated liquid. The mass flow rate of steam through the boiler is 24 kg/s. Show the cycle on a T-s diagram, and determine (a) the net power output of the power plant and (b) the thermal efficiency of the cycle.
(a)
The power output of plant.
Answer to Problem 52P
The power output of the plant is
Explanation of Solution
Draw the schematic diagram of the given ideal regenerative Rankine cycle as shown in
Figure 1.
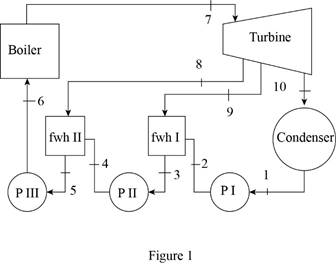
Draw the
Figure 2.
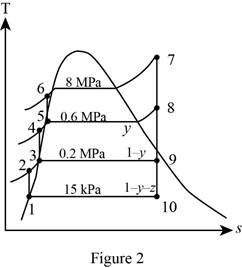
Here, water (steam) is the working fluid of the regenerative Rankine cycle. The cycle involves three pumps.
Write the formula for work done by the pump during process 1-2.
Here, the specific volume is
Write the formula for enthalpy
Write the formula for work done by the pump during process 3-4.
Here, the specific volume is
Write the formula for enthalpy
Write the formula for work done by the pump during process 5-6.
Here, the specific volume is
Write the formula for enthalpy
At state 9:
The steam expanded to the pressure of
The quality of water at the exit of the turbine (state 9) is expressed as follows.
The enthalpy at state 9 is expressed as follows.
Here, the enthalpy is
At state 10:
The steam enters the condenser at the pressure of
The quality of water at state 10 is expressed as follows.
The enthalpy at state 10 is expressed as follows.
Here, the subscript 10 indicates the process state 10.
Refer Figure 1 and 2.
Write the formula for heat in
Here, the mass fraction steam extracted from the turbine to the feed water entering the boiler via feed water heater-I
Write the general equation of energy balance equation.
Here, the rate of net energy inlet is
At steady state the rate of change of net energy of the system
Refer Equation (XIII).
Write the energy balance equation for open feed water heater-II.
Rewrite the Equation (XIV) in terms of mass fraction
Refer Equation (XIII).
Write the energy balance equation for open feed water heater-I.
Rewrite the Equation (XVI) in terms of mass fraction
Write the formula for net power output of the cycle per unit mass.
Write the formula for net power output of the cycle.
Here, the mass flow rate is
At state 1: (Pump I inlet)
The water exits the condenser as a saturated liquid at the pressure of
Refer Table A-5, “Saturated water-Pressure table”.
The enthalpy
At state 3: (Pump II inlet)
The water exits the open feed water heater-I as a saturated liquid at the pressure of
Refer Table A-5, “Saturated water-Pressure table”.
The enthalpy
At state 5: (Pump III inlet)
The water exits the open feed water heater-II as a saturated liquid at the pressure of
Refer Table A-5, “Saturated water-Pressure table”.
The enthalpy
At state 7:
The steam enters the turbine as superheated vapor.
Refer Table A-6, “Superheated water”.
The enthalpy
From Figure 2.
At state 8:
The steam expanded to the pressure of
Refer Table A-6, “Superheated water”.
The enthalpy
At state 9:
The steam expanded to the pressure of
Refer Table A-5, “Saturated water-Pressure table”.
Obtain the following properties corresponding to the pressure of
At state 10:
The steam enters the condenser at the pressure of
Refer Table A-5, “Saturated water-Pressure table”.
Obtain the following properties corresponding to the pressure of
Conclusion:
Substitute
Substitute
Substitute
Equation (III).
Substitute
Substitute
Equation (V).
Substitute
From Figure 1.
Substitute
Substitute
Equation (VIII).
Substitute
Substitute
Equation (X).
Consider the open feed water heater-II alone.
Substitute
Consider the open feed water heater-I alone.
Substitute
Substitute
Substitute
Substitute
Substitute
Thus, the power output of the plant is
(b)
The thermal efficiency of the cycle.
Answer to Problem 52P
The thermal efficiency of the cycle is
Explanation of Solution
Write the formula for thermal efficiency of the cycle
Conclusion:
Substitute
Thus, the thermal efficiency of the cycle is
Want to see more full solutions like this?
Chapter 10 Solutions
Thermodynamics: An Engineering Approach
- Please help me with this problem and show the full solution.arrow_forwardConsider a steam power plant that operates on an ideal regenerative Rankine cycle and has a net power output of 150 MW. Steam enters the turbine at 10 MPa and 500°C and the condenser at 15 kPa. Steam is extracted from the turbine at 0.5 MPa to heat the feedwater in an open feedwater heater. Water leaves the feedwater heater as a saturated liquid. Show the cycle on a T-s diagram, and determine (a) the mass flow rate of steam through the boiler, and (b) the thermal efficiency of the cycle.arrow_forwardConsider a 150-MW steam power plant that operates on a simple Rankine cycle. Steam enters the turbine at 7 MPa and 500°C and is cooled in the condenser at 10 kPa. Calculate the mass flow rate of steam produced by the boiler. Assume an isentropic efficiency of 87% for both the turbine and the pump.arrow_forward
- A steam power plant operates on an ideal regenerative Rankine cycle. Steam enters the turbine at 6 MPa and 450°C and is condensed in the condenser at 20 kPa. Steam is extracted from the turbine at 0.4 MPa to heat the feedwater in closed feedwater heater. Assume that the feedwater leaves the heater at the condensation temperature of the extracted steam and that the extracted steam leaves the heater as a saturated liquid and is pumped to the line carrying the feedwater. Show the cycle on a T-s diagram, and determine: (a) the net work output per kg of steam flowing through the boiler, and (b) the thermal efficiency of the cycle.arrow_forwardAn ideal reheat 210 MW Rankine cycle with water as the working fluid operates the boiler at 15 MPa, the reheater at 2 MPa, and the condenser at 10 kPa. The temperature is 500 0C at the entrance of the high-pressure and low-pressure turbines. Determine (a) the quality of the steam at the low-pressure turbine exit, (b) the thermal efficiency of this system, and (c) the rate of heat transfer in the reheater.arrow_forwardConsider a 150-MW steam power plant that operates on a simple Rankine cycle. Steam enters the turbine at 7 MPa and 500°C and is cooled in the condenser at 10 kPa. Calculate the volume flow rate of sea water (S.G. = 1.05) used in the condenser, if the allowable temperature rise is 5°C. Assume an isentropic efficiency of 87% for both the turbine and the pump.arrow_forward
- Consider an ideal steam regenerative Rankine cycle with two feedwater heaters, one closed and one open. Steam enters the turbine at 10 MPa and 500 C and exhausts to the condenser at 10 kPa. Steam is extracted from the turbine at 0.7 MPa for the closed feedwater heater and 0.3 MPa for the open one. The extracted steam leaves the closed feedwater heater and is subsequently throttled to the open feedwater heater. Show the cycle on a T-s diagram with respect to saturation lines, and using only the data presented in the data tables given below determine a) the fraction for steam leaving the boiler that is extracted at 0.3 MPa z=0.1425 b) the fraction of steam leaving the boiler that is extracted at 0.7 MPa y=0.06213 c) the heat transfer from the condenser per unit mass leaving the boiler q_out=1509 kJ/kg d) the heat transfer to the boiler per unit mass leaving the boiler qin=2677 kJ/kg e) the mass flow rate of steam through the boiler for a net power output of 250 MW m'=214.1 kg/s f) the…arrow_forwardConsider a steam power plant that operates on a reheat Rankine cycle and has a net power output of 80 MW. Steam enters the high-pressure turbine at 10 MPa and 500°C and the low-pressure turbine at 1.4 MPa and 500°C. Steam leaves the condenser as a saturated liquid at a pressure of 10 kPa. Assume both turbine and compressor are isentropic. Show the cycle on a T-s diagram with respect to saturation lines.arrow_forwardThe net power of a steam power plant operating on the simple ideal Rankine cycle is 30.5 MW. Water vapor enters the turbine at a pressure of 7 MPa and a temperature of 500 °C, and expands to the pressure of the 10 kShare condenser in the turbine. In the steam condenser, it is cooled and condensed with water supplied from a lake. The flow rate of the lake water is 1950 kg/s. Take the adiabatic efficiency of the pump and turbine by 87%. Show the cycle in the T-sdiagram. Csu=4.18kJ/kg°C a) The thermal efficiency of the cycle,b) The flow of steam circulating in the circuit,c)Calculate the temperature rise of the cooling water.arrow_forward
- A steam power plant operates on an ideal regenerative Rankine cycle. Steam enters the turbine at 6 MPa and 450°C and is condensed in the condenser at 20 kPa. Steam is extracted from the turbine at 0.4 MPa to heat the feed-water in an open feed- water heater. Water leaves the feed-water heater as a saturated liquid. Show the cycle on a T-s diagram, and determine (a) the net work output per kilogram of steam flowing through the boiler and, (b) the thermal efficiency of the cycle.arrow_forwardA steam power plant operates on an ideal regenerative Rankine cycle. Steam enters the turbine at 6 MPa and 450°C and is condensed in the condenser at 20 kPa. Steam is extracted from the turbine at 0.4 MPa to heat the feedwater in an open feedwater heater. Water leaves the feedwater heater as a saturated liquid. Show the cycle on a T-s diagram, and determine: (a) the net work output per kg of steam, and (b) the thermal efficiency of the cycle.arrow_forwardSteam enters the turbine of a steam power plant that operates on a simple ideal Rankine cycle at a pressure of 6 MPa, and it leaves as a saturated vapor at 7.5 kPa. Heat is transferred to the steam in the boiler at a rate of 40,000 kJ/s. Steam is cooled in the condenser by the cooling water from a nearby river, which enters the condenser at 158C. Show the cycle on a T-s diagram with respect to saturation lines, and determine (a) the turbine inlet temperature, (b) the net power output and thermal efficiency, and (c) the minimum mass flow rate of the cooling water required.arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





