
(a)
Interpretation:
The stereo isomeric product should be given, when it is reacts with per oxyacid followed by hydroxide ion.
Concept introduction:
Formation of
The
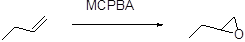
In the nucleophilic substitution reaction, the
In
Reactant and nucleophile are present at the rate determination step.
The order of species involving in
Tertiary < Secondary < Primary
(b)
Interpretation:
The stereo isomeric product should be given, when it is reacts with per oxyacid followed by hydroxide ion.
Formation of epoxide:
The alkene can be converted to epoxide, when it is treated with MCPBA (m-chloro perbenzoic acid)
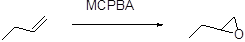
In the nucleophilic substitution reaction, the rate of reaction depends on reactant as well as nucleophile, which are involved in reaction is called bimolecular nucleophilic substitution reaction.
In
Reactant and nucleophile are present at the rate determination step.
The order of species involving in
Tertiary < Secondary < Primary
(c)
Interpretation:
The stereo isomeric product should be given, when it is reacts with per oxyacid followed by hydroxide ion.
Formation of epoxide:
The alkene can be converted to epoxide, when it is treated with MCPBA (m-chloro perbenzoic acid)
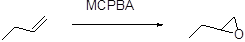
In the nucleophilic substitution reaction, the rate of reaction depends on reactant as well as nucleophile, which are involved in reaction is called bimolecular nucleophilic substitution reaction.
In
Reactant and nucleophile are present at the rate determination step.
The order of species involving in
Tertiary < Secondary < Primary
(d)
Interpretation:
The stereo isomeric product should be given, when it is reacts with per oxyacid followed by hydroxide ion.
Formation of epoxide:
The alkene can be converted to epoxide, when it is treated with MCPBA (m-chloro perbenzoic acid)
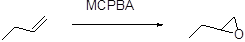
In the nucleophilic substitution reaction, the rate of reaction depends on reactant as well as nucleophile, which are involved in reaction is called bimolecular nucleophilic substitution reaction.
In
Reactant and nucleophile are present at the rate determination step.
The order of species involving in
Tertiary < Secondary < Primary
Want to see the full answer?
Check out a sample textbook solution
Chapter 10 Solutions
EBK ORGANIC CHEMISTRY
- a. H3C CH3 H, 1.0 equiv. Br2arrow_forwardH3C. H3C CH 3 CH 3 CH3 1. LDA 2. PhSeCl 3. H2O2arrow_forwardPlease predict the products for each of the following reactions: 1.03 2. H₂O NaNH, 1. n-BuLi 2. Mel A H₂ 10 9 0 H2SO4, H₂O HgSO4 Pd or Pt (catalyst) B 9 2 n-BuLi ♡ D2 (deuterium) Lindlar's Catalyst 1. NaNH2 2. EtBr Na, ND3 (deuterium) 2. H₂O2, NaOH 1. (Sia)2BH с Darrow_forward
- in the scope of ontario SCH4U grade 12 course, please show ALL workarrow_forwardIs the chemical reaction CuCl42-(green) + 4H2O <==> Cu(H2O)42+(blue) + 4Cl- exothermic or endothermic?arrow_forwardIf we react tetraethoxypropane with hydrazine, what is the product obtained (explain its formula). State the reason why the corresponding dialdehyde is not used.arrow_forward
- drawing, no aiarrow_forwardIf CH3COCH2CH(OCH3)2 (4,4-dimethoxy-2-butanone) and hydrazine react, two isomeric products are formed. State their structure and which will be the majority.arrow_forward+ Reset Provide the correct IUPAC name for the compound shown here. 4-methylhept-2-ene (Z)- (E)- 1-6-5-2-3-4- cyclo iso tert- sec- di tri hept hex oct meth eth pent ane yne ene ylarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





