
EBK ORGANIC CHEMISTRY
8th Edition
ISBN: 8220102744127
Author: Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10, Problem 95P
Two stereoisomers are obtained from the reaction or cyclopentene oxide and dimethylmine. The R,R-isomer is used in the manufacture of eclanamine, an antidepressant. What other isomer is obtained?
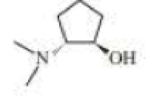
R,R-isomer
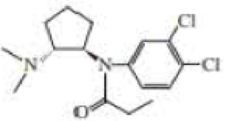
ecianamine
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
can you please help me solve hw
Give a brief comparison of chemical potential and electrochemical potential.
can you please help me solve hw
Chapter 10 Solutions
EBK ORGANIC CHEMISTRY
Ch. 10.1 - Why are NH3 and CH3NH2 no longer nucleophiles when...Ch. 10.1 - Explain the difference in reactivity between...Ch. 10.1 - Prob. 5PCh. 10.1 - Prob. 6PCh. 10.1 - Prob. 8PCh. 10.2 - Prob. 9PCh. 10.3 - Prob. 11PCh. 10.3 - Show how 1-propanol can be converted into the...Ch. 10.4 - Which of the following alcohols dehydrates the...Ch. 10.4 - Prob. 14P
Ch. 10.4 - Prob. 15PCh. 10.4 - Propose a mechanism for each of the following...Ch. 10.4 - Draw the product of each of the following...Ch. 10.4 - Explain why the following alcohols, when heated...Ch. 10.4 - What stereoisomers are formed in the following...Ch. 10.4 - Prob. 20PCh. 10.4 - What alcohol would you treat with phosphorus...Ch. 10.5 - Prob. 22PCh. 10.6 - What are the major products obtained when each of...Ch. 10.6 - Prob. 26PCh. 10.7 - Prob. 27PCh. 10.7 - Would you expect the reactivity of a five-membered...Ch. 10.7 - Prob. 29PCh. 10.7 - What products are obtained from the reaction of...Ch. 10.7 - Prob. 31PCh. 10.7 - Prob. 32PCh. 10.7 - Prob. 33PCh. 10.8 - Draw the mechanism for formation of the two...Ch. 10.8 - Prob. 35PCh. 10.8 - Prob. 36PCh. 10.8 - How do the major products obtained from...Ch. 10.8 - Explain why the two arene oxides in Problem 38...Ch. 10.8 - Which compound is more likely to be carcinogenic?Ch. 10.8 - Three arene oxides can be obtained from...Ch. 10.9 - Explain why the half-life (the time it takes for...Ch. 10.10 - Prob. 43PCh. 10.10 - Prob. 44PCh. 10.10 - Prob. 45PCh. 10.10 - Prob. 46PCh. 10.10 - Prob. 47PCh. 10.10 - Describe a synthesis for each of the following...Ch. 10.11 - Using an alkyl halide and a thiol as starting...Ch. 10.11 - The following three nitrogen mustards were studied...Ch. 10.11 - Why is melphalan a good cancer drug?Ch. 10.11 - Prob. 53PCh. 10.12 - Propose a mechanism for the following reaction:Ch. 10 - Prob. 55PCh. 10 - Which compound is more likely to be carcinogenic?Ch. 10 - Prob. 57PCh. 10 - Prob. 58PCh. 10 - When heated with H2SO4, both...Ch. 10 - What is the major product obtained from the...Ch. 10 - Write the appropriate reagent over each arrow.Ch. 10 - What alkenes would you expect to be obtained from...Ch. 10 - Prob. 63PCh. 10 - Prob. 64PCh. 10 - When deuterated phenanthrene oxide undergoes a...Ch. 10 - An unknown alcohol with a molecular formula of...Ch. 10 - Explain why the acid-catalyzed dehydration of an...Ch. 10 - Prob. 68PCh. 10 - Prob. 69PCh. 10 - Propose a mechanism for the following reaction:Ch. 10 - What product would be formed if the four-membered...Ch. 10 - Which of the following ethers would be obtained in...Ch. 10 - Using the given starting material any necessary...Ch. 10 - Prob. 74PCh. 10 - When 3-methyl-2-butanol is heated with...Ch. 10 - Draw structures for compounds AF.Ch. 10 - Propose a mechanism for each of the following...Ch. 10 - How could you synthesize isopropyl propyl ether,...Ch. 10 - When ethyl ether is heated with excess HI for...Ch. 10 - When the following seven-membered ring alcohol is...Ch. 10 - Ethylene oxide reacts readily with HO because of...Ch. 10 - Describe how each of the following compounds could...Ch. 10 - Propose a mechanism for each of the following...Ch. 10 - Triethylene glycol is one of the products obtained...Ch. 10 - Prob. 85PCh. 10 - Propose a mechanism for the following reaction:Ch. 10 - Prob. 87PCh. 10 - An ion with a positively charged nitrogen atom in...Ch. 10 - The following reaction takes place several times...Ch. 10 - Prob. 90PCh. 10 - Propose a mechanism for each of the following...Ch. 10 - A vicinal diol has OH groups on adjacent carbons....Ch. 10 - Prob. 93PCh. 10 - Prob. 94PCh. 10 - Two stereoisomers are obtained from the reaction...Ch. 10 - Propose a mechanism for each or the following...Ch. 10 - Triethylenemelamine (TEM) is an antitumor agent....
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Reason whether it is possible to determine changes in the Galvani potential difference at the metal-solution interface.arrow_forwardObtain the standard potential at 25°C of the Cu* I Cu | Pt electrode from the standard potentials E° Cu²+/Cu = 0.341 V and E Cu²+ /Cu+ = 0.153 V.arrow_forwardIn electrochemistry, briefly describe the Galvani potential, the Volta potential, and the surface potential. Differentiate between them.arrow_forward
- What substances can neutralize, complex or adsorb and absorb both HF and CF carbonyl fluoride and hydrogen fluoride and intermediate formation of thermal decomposition of fluorinated inorganic compounds either due to hydrolysis and hygroscopic reactions. What is the known chemistry of these reactions and mechanisms.arrow_forwardBriefly differentiate between chemical potential and electrochemical potential.arrow_forwardAccording to open access forums ionic antimony Sb (111) can be reduced to elemental Sb (0) in solution and in macromolecules like condensation polymers polyethylene terephthalate (PET) causing greying of the polymer matrix. It has been connected to thermal degradation of the polymer during processing to the formation of thermally unstable EG ethyleen glycol that forms at various temperatures formic acid, formaldehyde, acetaldehyde and much more depending on temperature. I need to know what organics are more powerful reducing agents and at what concentration (relative) to each organic will initiate this reduction. Furthermore, is the pH dependant ? Are other trace elements in the plastic also a cause of concern e.g. aluminum from aluminum chloride (lewis acid). Therefore, the ultimate solution should include a means to inhibit reduction of ionic antimony and will the same solution comply with cobalt impurities from ionic cobalt? Some PET have combinations of catalyst and their residues…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License