
(a)
Interpretation:
The mechanism of the given reaction should be proposed.
Concept introduction:
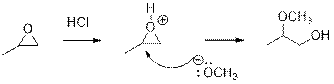
In the presence of acid catalyst, this reaction takes place through partial
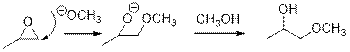
Epoxides are reactive, methoxide ion attacks epoxides in a less sterically hindered position which forms the alkoxide ion, and then it gets proton from alcohol which form the product.
(b)
Interpretation:
The structure of all products that formed in the given reaction should be drawn.
Concept introduction:

In the presence of acid catalyst, this reaction takes place through partial
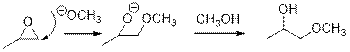
Epoxides are reactive, methoxide ion attacks epoxides in a less sterically hindered position which forms the alkoxide ion, and then it gets proton from alcohol which form the product.
(c)
Interpretation:
The reason should be explained for the formation of six member ring compound in the given reaction.
Concept introduction:

In the presence of acid catalyst, this reaction takes place through partial
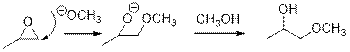
Epoxides are reactive, methoxide ion attacks epoxides in a less sterically hindered position which forms the alkoxide ion, and then it gets proton from alcohol which form the product.
Want to see the full answer?
Check out a sample textbook solution
Chapter 10 Solutions
EBK ORGANIC CHEMISTRY
- HELP NOW PLEASE ! ASAP! URGENT!arrow_forwardHELP NOW PLEASE ! ASAP! URGENT!arrow_forwardDraw a Newman projection for the molecule below from the perspective indicated. Which of the groups (letters A-H) are methyl groups? CH3 H H H A H B ☑ >> H. ABCDEFG I H -H CH3 G D CH F E Numeric 4 points How many gauche interactions exist in the conformation shown in the previous problem? 1arrow_forward
- HELP NOW PLEASE ! ASAP! URGENT!arrow_forwardHELP NOW PLEASE ! ASAP! URGENT!arrow_forwardWould the following organic synthesis occur in one step? Add any missing products, required catalysts, inorganic reagents, and other important conditions. Please include a detailed explanation and drawings showing how the reaction may occur in one step.arrow_forward
- Pls help.arrow_forward13) When solid barium phosphate is in equilibrium with its ions, the ratio of barium ions to phosphate ions would be: a. 1:1 b. 2:3 c. 3:2 d. 2:1 14) The pH of a 0.05 M solution of HCl(aq) at 25°C is 15) The pH of a 0.20 M solution of KOH at 25°C isarrow_forwardPls help.arrow_forward
- Pls help.arrow_forward16) A 2.0 L flask containing 2.0 x 10-3 mol H2(g), 3.0 x 10-3 mol Cl2(g), and 4.0 x 10-3 mol HCl(g) at equilibrium. This system is represented by the following chemical equation: H2 (g) + Cl2 (g) → 2HCl(g) Calculate the equilibrium constant for this reaction.arrow_forward7) The pH of a 0.05M solution of HCl(aq) at 25°C is a. 1.3 b. 2.3 c. 3.3 d. 12.7arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
