
Bundle: Introductory Chemistry: An Active Learning Approach, 6th + LMS Integrated for OWLv2, 4 terms (24 months) Printed Access Card
6th Edition
ISBN: 9781305717428
Author: Mark S. Cracolice, Ed Peters
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10, Problem 41E
Ammonia can be formed from a combination reaction of its elements. A small fraction of an unreacted mixture of elements is illustrated in the following diagram, in which white spheres represent hydrogen atoms and blue spheres represent nitrogen atoms. The temperature is such that all species are gases.
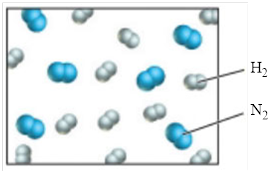
a) Write and balance the equation for the reaction.
b) Which of the following correctly represents the product mixture?
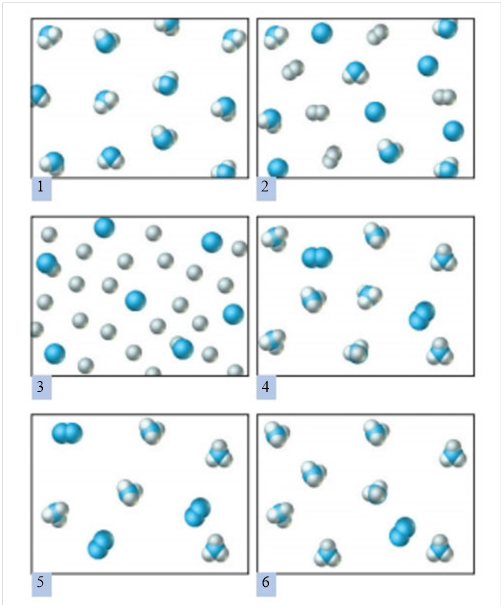
c) Which species is the limiting reactant? Explain.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
A certain half-reaction has a standard reduction potential Ered +1.26 V. An engineer proposes using this half-reaction at the anode of a galvanic cell that
must provide at least 1.10 V of electrical power. The cell will operate under standard conditions.
Note for advanced students: assume the engineer requires this half-reaction to happen at the anode of the cell.
Is there a minimum standard reduction
potential that the half-reaction used at
the cathode of this cell can have?
If so, check the "yes" box and calculate
the minimum. Round your answer to 2
decimal places. If there is no lower
limit, check the "no" box..
Is there a maximum standard reduction
potential that the half-reaction used at
the cathode of this cell can have?
If so, check the "yes" box and calculate
the maximum. Round your answer to 2
decimal places. If there is no upper
limit, check the "no" box.
yes, there is a minimum.
1
red
Πν
no minimum
Oyes, there is a maximum.
0
E
red
Dv
By using the information in the ALEKS…
In statistical thermodynamics, check the
hcv
following equality: ß Aɛ =
KT
Please correct answer and don't used hand raiting
Chapter 10 Solutions
Bundle: Introductory Chemistry: An Active Learning Approach, 6th + LMS Integrated for OWLv2, 4 terms (24 months) Printed Access Card
Ch. 10 - The first step in the Ostwald process for...Ch. 10 - When hydrogen sulfide reacts with oxygen, water...Ch. 10 - Magnesium hydroxide is formed from the reaction of...Ch. 10 - In our bodies, sugar is broken down by reacting...Ch. 10 - Prob. 5ECh. 10 - Aqueous solutions of potassium hydrogen sulfate...Ch. 10 - The first step in the Ostwald process for...Ch. 10 - Butane, C4H10 is a common fuel used for heating...Ch. 10 - The explosion of nitroglycerine is described by...Ch. 10 - According to the reaction 2AgNO3+CuCu(NO3)2+2Ag,...
Ch. 10 - Prob. 11ECh. 10 - Prob. 12ECh. 10 - Prob. 13ECh. 10 - Prob. 14ECh. 10 - The hard water scum that forms a ring around the...Ch. 10 - Prob. 16ECh. 10 - Prob. 17ECh. 10 - Prob. 18ECh. 10 - The Solvay process is multistep industrial method...Ch. 10 - Prob. 20ECh. 10 - Prob. 21ECh. 10 - What mass of NaHCO3 must decompose to produce 448g...Ch. 10 - Prob. 23ECh. 10 - Solid ammonium chloride decomposes to form ammonia...Ch. 10 - What mass of magnesium hydroxide will precipitate...Ch. 10 - Prob. 26ECh. 10 - Prob. 27ECh. 10 - Prob. 28ECh. 10 - The reaction of a dry cell battery may be...Ch. 10 - Prob. 30ECh. 10 - Prob. 31ECh. 10 - Prob. 32ECh. 10 - Calcium cyanamide is a common fertilizer. When...Ch. 10 - Prob. 34ECh. 10 - The Haber process for making ammonia from nitrogen...Ch. 10 - Prob. 36ECh. 10 - Prob. 37ECh. 10 - The simplest example of the hydrogenation of a...Ch. 10 - Prob. 39ECh. 10 - Prob. 40ECh. 10 - Ammonia can be formed from a combination reaction...Ch. 10 - Carbon monoxide reacts with oxygen to form carbon...Ch. 10 - An experiment is conducted in which varying...Ch. 10 - The flasks below illustrated three trials of a...Ch. 10 - A solution containing 1.63g of barium chloride is...Ch. 10 - Prob. 46ECh. 10 - Prob. 47ECh. 10 - Prob. 48ECh. 10 - A mixture of tetraphosphorus trisulfide and...Ch. 10 - Sodium carbonate can neutralize nitric acid by the...Ch. 10 - Prob. 51ECh. 10 - Prob. 52ECh. 10 - Prob. 53ECh. 10 - Prob. 54ECh. 10 - Prob. 55ECh. 10 - Prob. 56ECh. 10 - Prob. 57ECh. 10 - Prob. 58ECh. 10 - Prob. 59ECh. 10 - Prob. 60ECh. 10 - Question 57 through 62: Thermochemical equations...Ch. 10 - Prob. 62ECh. 10 - Quicklime, the common name for calcium oxide, CaO,...Ch. 10 - What mass in grams of hydrogen has to react to...Ch. 10 - The quicklime produced in Question 63 is...Ch. 10 - Prob. 66ECh. 10 - What mass in grams of octane, a component of...Ch. 10 - Calculate the quantity of energy (kJ) transferred...Ch. 10 - Prob. 69ECh. 10 - Classify each of the following statements as true...Ch. 10 - Prob. 71ECh. 10 - What mass in grams of calcium phosphate will...Ch. 10 - Prob. 73ECh. 10 - Prob. 74ECh. 10 - A laboratory test of 12.8g of aluminum ore yields...Ch. 10 - How much energy is required to decompose 1.42g of...Ch. 10 - Prob. 77ECh. 10 - Prob. 78ECh. 10 - A sludge containing silver chloride is a water...Ch. 10 - Prob. 80ECh. 10 - Prob. 81ECh. 10 - Prob. 82ECh. 10 - Prob. 83ECh. 10 - Prob. 84ECh. 10 - In 1866, a young chemistry student conceived the...Ch. 10 - Prob. 86ECh. 10 - A student was given a 1.6240-g sample of a mixture...Ch. 10 - A researcher dissolved 1.382g of impure copper in...Ch. 10 - What mass in grams of magnesium nitrate, Mg(NO3)2,...Ch. 10 - Prob. 90ECh. 10 - Prob. 10.1TCCh. 10 - Solutions of zinc bromide and sodium hydroxide are...Ch. 10 - Prob. 2PECh. 10 - Prob. 3PECh. 10 - How mass of fluorine is formed when 3.0grams of...Ch. 10 - Prob. 5PECh. 10 - Prob. 6PECh. 10 - Prob. 7PECh. 10 - Prob. 8PECh. 10 - Prob. 9PECh. 10 - A solution containing 43.5g of calcium nitrate is...Ch. 10 - Prob. 11PECh. 10 - Prob. 12PECh. 10 - Prob. 13PECh. 10 - Prob. 14PECh. 10 - Prob. 15PECh. 10 - Prob. 1PCECh. 10 - Prob. 2PCECh. 10 - Prob. 3PCECh. 10 - Prob. 4PCECh. 10 - Prob. 5PCECh. 10 - Prob. 6PCECh. 10 - Eight problem-classification examples follow. Test...Ch. 10 - Prob. 8PCE
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please correct answer and don't used hand raitingarrow_forward(11pts total) Consider the arrows pointing at three different carbon-carbon bonds in the molecule depicted below. Bond B Bond A Bond C a. (2pts) Which bond between A-C is weakest? Which is strongest? Place answers in appropriate boxes. Weakest Bond Strongest Bond b. (4pts) Consider the relative stability of all cleavage products that form when bonds A, B, AND C are homolytically cleaved/broken. Hint: cleavage products of bonds A, B, and C are all carbon radicals. i. Which ONE cleavage product is the most stable? A condensed or bond line representation is fine. ii. Which ONE cleavage product is the least stable? A condensed or bond line representation is fine. c. (5pts) Use principles discussed in lecture, supported by relevant structures, to succinctly explain the why your part b (i) radical is more stable than your part b(ii) radical. Written explanation can be no more than one-two succinct sentence(s)!arrow_forward. 3°C with TH 12. (10pts total) Provide the major product for each reaction depicted below. If no reaction occurs write NR. Assume heat dissipation is carefully controlled in the fluorine reaction. 3H 24 total (30) 24 21 2h • 6H total ● 8H total 34 래 Br2 hv major product will be most Substituted 12 hv Br NR I too weak of a participate in P-1 F₂ hv Statistically most favored product will be major = most subst = thermo favored hydrogen atom abstractor to LL Farrow_forward
- Five chemistry project topic that does not involve practicalarrow_forwardPlease correct answer and don't used hand raitingarrow_forwardQ2. Consider the hydrogenation of ethylene C2H4 + H2 = C2H6 The heats of combustion and molar entropies for the three gases at 298 K are given by: C2H4 C2H6 H2 AH comb/kJ mol¹ -1395 -1550 -243 Sº / J K¹ mol-1 220.7 230.4 131.1 The average heat capacity change, ACP, for the reaction over the temperature range 298-1000 K is 10.9 J K¹ mol¹. Using these data, determine: (a) the standard enthalpy change at 800 K (b) the standard entropy change at 800 K (c) the equilibrium constant at 800 K.arrow_forward
- 13. (11pts total) Consider the arrows pointing at three different carbon-carbon bonds in the molecule depicted below. Bond B Bond A Bond C a. (2pts) Which bond between A-C is weakest? Which is strongest? Place answers in appropriate boxes. Weakest Bond Strongest Bond b. (4pts) Consider the relative stability of all cleavage products that form when bonds A, B, AND C are homolytically cleaved/broken. Hint: cleavage products of bonds A, B, and C are all carbon radicals. i. Which ONE cleavage product is the most stable? A condensed or bond line representation is fine. ii. Which ONE cleavage product is the least stable? A condensed or bond line representation is fine. c. (5pts) Use principles discussed in lecture, supported by relevant structures, to succinctly explain the why your part b (i) radical is more stable than your part b(ii) radical. Written explanation can be no more than one-two succinct sentence(s)! Googlearrow_forwardPrint Last Name, First Name Initial Statifically more chances to abstract one of these 6H 11. (10pts total) Consider the radical chlorination of 1,3-diethylcyclohexane depicted below. 4 4th total • 6H total 래 • 4H total 21 total ZH 2H Statistical H < 3° C-H weakest - product abstraction here bund leads to thermo favored a) (6pts) How many unique mono-chlorinated products can be formed and what are the structures for the thermodynamically and statistically favored products? Product 6 Number of Unique Mono-Chlorinated Products Thermodynamically Favored Product Statistically Favored Product b) (4pts) Draw the arrow pushing mechanism for the FIRST propagation step (p-1) for the formation of the thermodynamically favored product. Only draw the p-1 step. You do not need to include lone pairs of electrons. No enthalpy calculation necessary H H-Cl Waterfoxarrow_forward10. (5pts) Provide the complete arrow pushing mechanism for the chemical transformation → depicted below Use proper curved arrow notation that explicitly illustrates all bonds being broken, and all bonds formed in the transformation. Also, be sure to include all lone pairs and formal charges on all atoms involved in the flow of electrons. CH3O II HA H CH3O-H H ①arrow_forward
- Do the Lone Pairs get added bc its valence e's are a total of 6 for oxygen and that completes it or due to other reasons. How do we know the particular indication of such.arrow_forwardNGLISH b) Identify the bonds present in the molecule drawn (s) above. (break) State the function of the following equipments found in laboratory. Omka) a) Gas mask b) Fire extinguisher c) Safety glasses 4. 60cm³ of oxygen gas diffused through a porous hole in 50 seconds. How long w 80cm³ of sulphur(IV) oxide to diffuse through the same hole under the same conditions (S-32.0.0-16.0) (3 m 5. In an experiment, a piece of magnesium ribbon was cleaned with steel w clean magnesium ribbon was placed in a crucible and completely burnt in oxy cooling the product weighed 4.0g a) Explain why it is necessary to clean magnesium ribbon. Masterclass Holiday assignmen PB 2arrow_forwardHi!! Please provide a solution that is handwritten. Ensure all figures, reaction mechanisms (with arrows and lone pairs please!!), and structures are clearly drawn to illustrate the synthesis of the product as per the standards of a third year organic chemistry course. ****the solution must include all steps, mechanisms, and intermediate structures as required. Please hand-draw the mechanisms and structures to support your explanation. Don’t give me AI-generated diagrams or text-based explanations, no wordy explanations on how to draw the structures I need help with the exact mechanism hand drawn by you!!! I am reposting this—ensure all parts of the question are straightforward and clear or please let another expert handle it thanks!!arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781285199030
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY