
a)
Interpretation:
The difference in the boiling points of the given set of compounds has to be rationalized.
Concept Introduction:
- Boiling point of any compound, depends upon its strength of Intramolecular force and Intermolecular force present in it.
- Intramolecular force refers to type of bonding between the atoms.
- Intermolecular forces are the forces that bind the molecules together to attribute to a stability of a compound.
- If the strength of intermolecular forces is high, boiling point will be high and if it is low, boiling point will be low.
- They are collectively known as “Interparticle forces”. The classification can be summarized as follows –
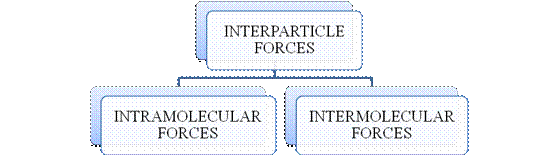
Figure 1
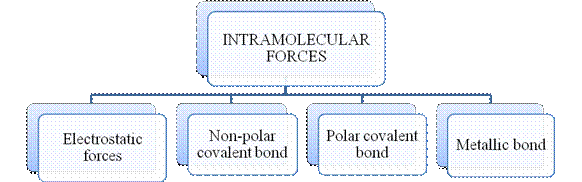
Figure 2
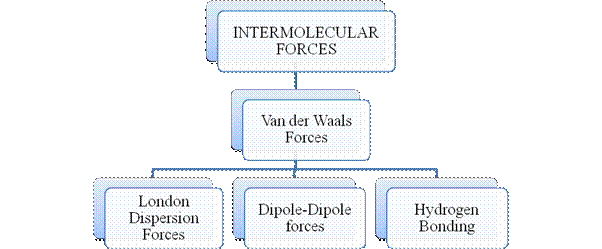
Figure 3
The type of bonding between atoms or ions is Intramolecular force. The intramolecular force in ionic compounds is electrostatic force of attraction between the ions of opposite charges. Usually ionic compounds are solids with high melting points. Covalent bonds are of two types, that is polar covalent bond and non-polar covalent bond. Covalent compounds are found as solids and liquids with moderate melting and boiling point. Metallic bond is formed between the metal atoms of an element.
Intermolecular forces are Van der Waals forces. They are weak and are of three types - London dispersion forces, dipole-dipole forces and hydrogen bonding. Hydrogen bonding is formed in polar covalent compounds containing hydrogen and other high electronegativity like fluorine, oxygen or nitrogen. These atoms in a molecule partially bond to hydrogen of the other same molecule or within a molecule. It is relatively the strongest one. Hydrogen bonded compounds are usually liquids. They exhibit high boiling point.
London dispersion forces exist in all types of molecules. This is the force responsible for the condensation of non-polar compounds into liquids or solids under low temperature.
Dipole-dipole forces exist in polar covalent compounds. Hydrogen bonding exists in polar covalent compounds containing Fluorine, Oxygen or Nitrogen directly bonded to Hydrogen.
b)
Interpretation:
The difference in the boiling points of the given set of compounds has to be rationalized.
Concept Introduction:
- Boiling point of any compound, depends upon its strength of Intramolecular force and Intermolecular force present in it.
- Intramolecular force refers to type of bonding between the atoms.
- Intermolecular forces are the forces that bind the molecules together to attribute to a stability of a compound.
- If the strength of intermolecular forces is high, boiling point will be high and if it is low, boiling point will be low.
- They are collectively known as “Interparticle forces”. The classification can be summarized as follows –
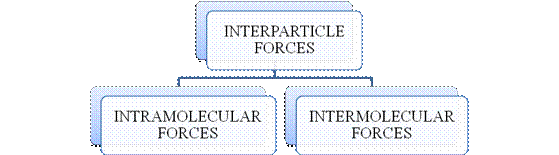
Figure 1
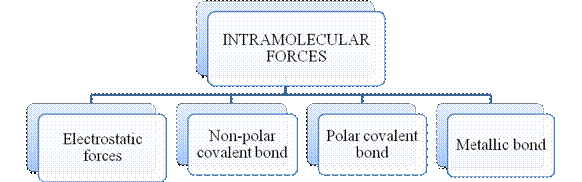
Figure 2
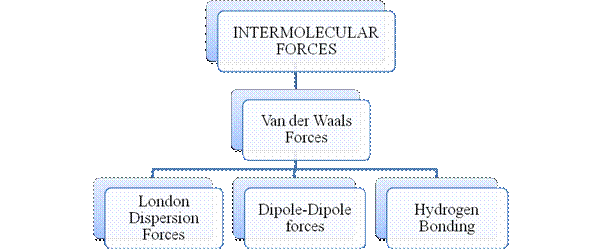
Figure 3
The type of bonding between atoms or ions is Intramolecular force. The intramolecular force in ionic compounds is electrostatic force of attraction between the ions of opposite charges. Usually ionic compounds are solids with high melting points. Covalent bonds are of two types, that is polar covalent bond and non-polar covalent bond. Covalent compounds are found as solids and liquids with moderate melting and boiling point. Metallic bond is formed between the metal atoms of an element.
Intermolecular forces are Van der Waals forces. They are weak and are of three types - London dispersion forces, dipole-dipole forces and hydrogen bonding. Hydrogen bonding is formed in polar covalent compounds containing hydrogen and other high electronegativity like fluorine, oxygen or nitrogen. These atoms in a molecule partially bond to hydrogen of the other same molecule or within a molecule. It is relatively the strongest one. Hydrogen bonded compounds are usually liquids. They exhibit high boiling point.
London dispersion forces exist in all types of molecules. This is the force responsible for the condensation of non-polar compounds into liquids or solids under low temperature.
Dipole-dipole forces exist in polar covalent compounds. Hydrogen bonding exists in polar covalent compounds containing Fluorine, Oxygen or Nitrogen directly bonded to Hydrogen.
c)
Interpretation:
The difference in the boiling points of the given set of compounds has to be rationalized.
Concept Introduction:
- Boiling point of any compound, depends upon its strength of Intramolecular force and Intermolecular force present in it.
- Intramolecular force refers to type of bonding between the atoms.
- Intermolecular forces are the forces that bind the molecules together to attribute to a stability of a compound.
- If the strength of intermolecular forces is high, boiling point will be high and if it is low, boiling point will be low.
- They are collectively known as “Interparticle forces”. The classification can be summarized as follows –
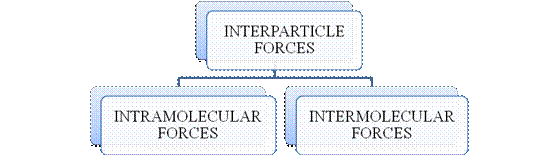
Figure 1

Figure 2
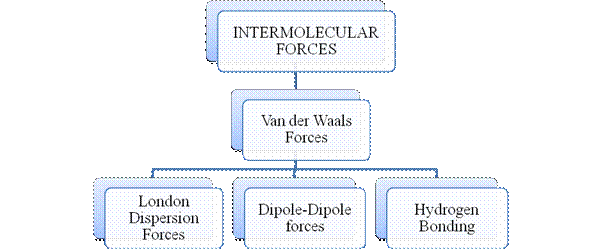
Figure 3
The type of bonding between atoms or ions is Intramolecular force. The intramolecular force in ionic compounds is electrostatic force of attraction between the ions of opposite charges. Usually ionic compounds are solids with high melting points. Covalent bonds are of two types, that is polar covalent bond and non-polar covalent bond. Covalent compounds are found as solids and liquids with moderate melting and boiling point. Metallic bond is formed between the metal atoms of an element.
Intermolecular forces are Van der Waals forces. They are weak and are of three types - London dispersion forces, dipole-dipole forces and hydrogen bonding. Hydrogen bonding is formed in polar covalent compounds containing hydrogen and other high electronegativity like fluorine, oxygen or nitrogen. These atoms in a molecule partially bond to hydrogen of the other same molecule or within a molecule. It is relatively the strongest one. Hydrogen bonded compounds are usually liquids. They exhibit high boiling point.
London dispersion forces exist in all types of molecules. This is the force responsible for the condensation of non-polar compounds into liquids or solids under low temperature.
Dipole-dipole forces exist in polar covalent compounds. Hydrogen bonding exists in polar covalent compounds containing Fluorine, Oxygen or Nitrogen directly bonded to Hydrogen.
d)
Interpretation:
The difference in the boiling points of the given set of compounds has to be rationalized.
Concept Introduction:
- Boiling point of any compound, depends upon its strength of Intramolecular force and Intermolecular force present in it.
- Intramolecular force refers to type of bonding between the atoms.
- Intermolecular forces are the forces that bind the molecules together to attribute to a stability of a compound.
- If the strength of intermolecular forces is high, boiling point will be high and if it is low, boiling point will be low.
- They are collectively known as “Interparticle forces”. The classification can be summarized as follows –
Figure 1

Figure 2

Figure 3
The type of bonding between atoms or ions is Intramolecular force. The intramolecular force in ionic compounds is electrostatic force of attraction between the ions of opposite charges. Usually ionic compounds are solids with high melting points. Covalent bonds are of two types, that is polar covalent bond and non-polar covalent bond. Covalent compounds are found as solids and liquids with moderate melting and boiling point. Metallic bond is formed between the metal atoms of an element.
Intermolecular forces are Van der Waals forces. They are weak and are of three types - London dispersion forces, dipole-dipole forces and hydrogen bonding. Hydrogen bonding is formed in polar covalent compounds containing hydrogen and other high electronegativity like fluorine, oxygen or nitrogen. These atoms in a molecule partially bond to hydrogen of the other same molecule or within a molecule. It is relatively the strongest one. Hydrogen bonded compounds are usually liquids. They exhibit high boiling point.
London dispersion forces exist in all types of molecules. This is the force responsible for the condensation of non-polar compounds into liquids or solids under low temperature.
Dipole-dipole forces exist in polar covalent compounds. Hydrogen bonding exists in polar covalent compounds containing Fluorine, Oxygen or Nitrogen directly bonded to Hydrogen.
Trending nowThis is a popular solution!

Chapter 10 Solutions
Student Solutions Manual for Zumdahl/Zumdahl/DeCoste?s Chemistry, 10th Edition
- theres 2 productsarrow_forwardDraw the major product of this solvolysis reaction. Ignore any inorganic byproducts. + CH3CH2OH Drawing Q Atoms, Bonds and Rings OCH2CH3 || OEt Charges OH 00-> | Undo Reset | Br Remove Done Drag To Pan +arrow_forwardDraw the major product of this SN1 reaction. Ignore any inorganic byproducts. CH3CO2Na CH3CO2H Drawing + Br Q Atoms, Bonds and Rings OAC Charges OH ОАс Na ဂ Br Undo Reset Remove Done Drag To Pan +arrow_forward
- Organic Functional Groups entifying positions labeled with Greek letters in acids and derivatives 1/5 ssible, replace an H atom on the a carbon of the molecule in the drawing area with a ce an H atom on the ẞ carbon with a hydroxyl group substituent. ne of the substituents can't be added for any reason, just don't add it. If neither substi er the drawing area. O H OH Oneither substituent can be added. Check D 1 Accessibility ado na witharrow_forwardDifferentiate between electrophilic and nucleophilic groups. Give examples.arrow_forwardAn aldehyde/ketone plus an alcohol gives a hemiacetal, and an excess of alcohol gives an acetal. The reaction is an equilibrium; in aldehydes, it's shifted to the right and in ketones, to the left. Explain.arrow_forward
- Draw a Haworth projection or a common cyclic form of this monosaccharide: H- -OH H- OH H- -OH CH₂OHarrow_forwardAnswer the question in the first photoarrow_forwardGgggffg2258555426855 please don't use AI Calculate the positions at which the probability of a particle in a one-dimensional box is maximum if the particle is in the fifth energy level and in the eighth energy level.arrow_forward
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning





