
Inquiry into Physics
8th Edition
ISBN: 9781337515863
Author: Ostdiek
Publisher: Cengage
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 10, Problem 13P
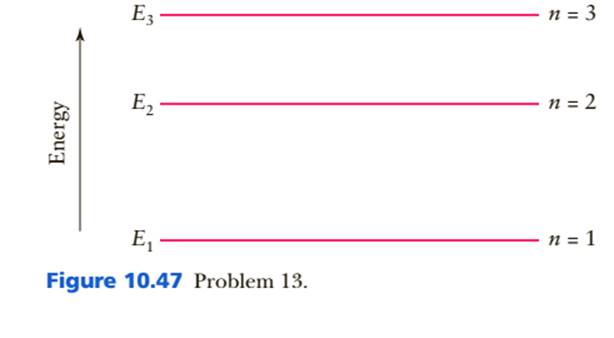
. Figure 10.47 is the energy-level diagram for a particularly simple, fictitious element, Vernium (Vn). Indicate by the use of arrows all allowed transitions leading to the emission of photons from this atom and order the frequencies of these photons from highest (largest) to lowest (smallest).
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Please solve and answer this problem correctly please. Thank you!!
Please solve and answer this problem correctly please. Thank you!!
a) Use the node-voltage method to find v1, v2, and
v3 in the circuit in Fig. P4.14.
b) How much power does the 40 V voltage source
deliver to the circuit?
Figure P4.14
302
202
w
w
+
+
+
40 V
V1
80 Ω 02
ΣΑΩ
28 A
V3 +
w
w
102
202
Chapter 10 Solutions
Inquiry into Physics
Ch. 10 - Prob. 1SACh. 10 - Prob. 1OACh. 10 - Prob. 1PIPCh. 10 - Prob. 1MIOCh. 10 - Prob. 2MIOCh. 10 - Prob. 1QCh. 10 - Prob. 2QCh. 10 - Prob. 3QCh. 10 - Prob. 4QCh. 10 - Prob. 5Q
Ch. 10 - Prob. 6QCh. 10 - Prob. 7QCh. 10 - Prob. 8QCh. 10 - Prob. 9QCh. 10 - Prob. 10QCh. 10 - Prob. 11QCh. 10 - (Indicates a review question, which means it...Ch. 10 - Prob. 13QCh. 10 - Prob. 14QCh. 10 - (Indicates a review question, which means it...Ch. 10 - Prob. 16QCh. 10 - Prob. 17QCh. 10 - Prob. 18QCh. 10 - Prob. 19QCh. 10 - Prob. 20QCh. 10 - Prob. 21QCh. 10 - Prob. 22QCh. 10 - Prob. 23QCh. 10 - Prob. 24QCh. 10 - Prob. 25QCh. 10 - Prob. 26QCh. 10 - Prob. 27QCh. 10 - Prob. 28QCh. 10 - Prob. 29QCh. 10 - Prob. 30QCh. 10 - Prob. 31QCh. 10 - Prob. 32QCh. 10 - Prob. 33QCh. 10 - Prob. 34QCh. 10 - Prob. 35QCh. 10 - Prob. 36QCh. 10 - Prob. 37QCh. 10 - Prob. 38QCh. 10 - Prob. 39QCh. 10 - Prob. 40QCh. 10 - Prob. 41QCh. 10 - Prob. 42QCh. 10 - Prob. 1PCh. 10 - Prob. 2PCh. 10 - Prob. 3PCh. 10 - Prob. 4PCh. 10 - Prob. 5PCh. 10 - Prob. 6PCh. 10 - Prob. 7PCh. 10 - Prob. 8PCh. 10 - Prob. 9PCh. 10 - Prob. 10PCh. 10 - Prob. 11PCh. 10 - Prob. 12PCh. 10 - . Figure 10.47 is the energy-level diagram for a...Ch. 10 - Prob. 14PCh. 10 - Prob. 15PCh. 10 - Prob. 16PCh. 10 - Prob. 17PCh. 10 - Prob. 18PCh. 10 - Prob. 19PCh. 10 - Prob. 20PCh. 10 - Prob. 21PCh. 10 - Prob. 22PCh. 10 - Prob. 23PCh. 10 - Prob. 1CCh. 10 - Prob. 2CCh. 10 - The rate at which solar wind particles enter the...Ch. 10 - Prob. 4CCh. 10 - Prob. 5CCh. 10 - Prob. 6C
Additional Science Textbook Solutions
Find more solutions based on key concepts
Define histology.
Fundamentals of Anatomy & Physiology (11th Edition)
1. Rub your hands together vigorously. What happens? Discuss the energy transfers and transformations that take...
College Physics: A Strategic Approach (3rd Edition)
An electric motor has an effective resistance of 32.0 and an inductive reactance of 45.0 when working under l...
Fundamentals of Physics Extended
How could you separate a mixture of the following compounds? The reagents available to you are water, either, 1...
Organic Chemistry (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Please solve and answer this problem correctly please. Thank you!!arrow_forwardYou're on an interplanetary mission, in an orbit around the Sun. Suppose you make a maneuver that brings your perihelion in closer to the Sun but leaves your aphelion unchanged. Then you must have Question 2 options: sped up at perihelion sped up at aphelion slowed down at perihelion slowed down at aphelionarrow_forwardThe force of the quadriceps (Fq) and force of the patellar tendon (Fp) is identical (i.e., 1000 N each). In the figure below angle in blue is Θ and the in green is half Θ (i.e., Θ/2). A) Calculate the patellar reaction force (i.e., R resultant vector is the sum of the horizontal component of the quadriceps and patellar tendon force) at the following joint angles: you need to provide a diagram showing the vector and its components for each part. a1) Θ = 160 degrees, a2) Θ = 90 degrees. NOTE: USE ONLY TRIGNOMETRIC FUNCTIONS (SIN/TAN/COS, NO LAW OF COSINES, NO COMPLICATED ALGEBRAIC EQUATIONS OR ANYTHING ELSE, ETC. Question A has 2 parts!arrow_forward
- The force of the quadriceps (Fq) and force of the patellar tendon (Fp) is identical (i.e., 1000 N each). In the figure below angle in blue is Θ and the in green is half Θ (i.e., Θ/2). A) Calculate the patellar reaction force (i.e., R resultant vector is the sum of the horizontal component of the quadriceps and patellar tendon force) at the following joint angles: you need to provide a diagram showing the vector and its components for each part. a1) Θ = 160 degrees, a2) Θ = 90 degrees. NOTE: USE DO NOT USE LAW OF COSINES, NO COMPLICATED ALGEBRAIC EQUATIONS OR ANYTHING ELSE, ETC. Question A has 2 parts!arrow_forwardNo chatgpt pls will upvotearrow_forwardThe force of the quadriceps (Fq) and force of the patellar tendon (Fp) is identical (i.e., 1000 N each). In the figure below angle in blue is Θ and the in green is half Θ (i.e., Θ/2). A) Calculate the patellar reaction force (i.e., R resultant vector is the sum of the horizontal component of the quadriceps and patellar tendon force) at the following joint angles: you need to provide a diagram showing the vector and its components for each part. a1) Θ = 160 degrees, a2) Θ = 90 degrees. NOTE: USE ONLY TRIGNOMETRIC FUNCTIONS (SIN/TAN/COS, NO LAW OF COSINES, NO COMPLICATED ALGEBRAIC EQUATIONS OR ANYTHING ELSE, ETC. Question A has 2 parts!arrow_forward
- ་ The position of a particle is described by r = (300e 0.5t) mm and 0 = (0.3t²) rad, where t is in seconds. Part A Determine the magnitude of the particle's velocity at the instant t = 1.5 s. Express your answer to three significant figures and include the appropriate units. v = Value Submit Request Answer Part B ? Units Determine the magnitude of the particle's acceleration at the instant t = 1.5 s. Express your answer to three significant figures and include the appropriate units. a = Value A ? Unitsarrow_forwardSolve and answer the question correctly please. Thank you!!arrow_forwardSolve and answer the question correctly please. Thank you!!arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Foundations of Astronomy (MindTap Course List)PhysicsISBN:9781337399920Author:Michael A. Seeds, Dana BackmanPublisher:Cengage Learning
Foundations of Astronomy (MindTap Course List)PhysicsISBN:9781337399920Author:Michael A. Seeds, Dana BackmanPublisher:Cengage Learning Stars and Galaxies (MindTap Course List)PhysicsISBN:9781337399944Author:Michael A. SeedsPublisher:Cengage Learning
Stars and Galaxies (MindTap Course List)PhysicsISBN:9781337399944Author:Michael A. SeedsPublisher:Cengage Learning
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning University Physics Volume 3PhysicsISBN:9781938168185Author:William Moebs, Jeff SannyPublisher:OpenStax
University Physics Volume 3PhysicsISBN:9781938168185Author:William Moebs, Jeff SannyPublisher:OpenStax

Foundations of Astronomy (MindTap Course List)
Physics
ISBN:9781337399920
Author:Michael A. Seeds, Dana Backman
Publisher:Cengage Learning

Stars and Galaxies (MindTap Course List)
Physics
ISBN:9781337399944
Author:Michael A. Seeds
Publisher:Cengage Learning


Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

University Physics Volume 3
Physics
ISBN:9781938168185
Author:William Moebs, Jeff Sanny
Publisher:OpenStax
Atomic Number, Atomic Mass, and the Atomic Structure | How to Pass ChemistryThe Nucleus: Crash Course Chemistry #1; Author: Crash Course;https://www.youtube.com/watch?v=FSyAehMdpyI;License: Standard YouTube License, CC-BY