
Concept explainers
(a)
Interpretation:
IUPAC name has to be determined for the given compound.
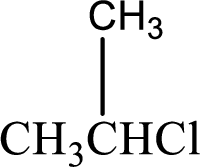
Concept Introduction:
A common nomenclature of naming organic compounds has been developed by IUPAC. By usage of this nomenclature or rules, memorizing of names of organic compounds is not necessary.
IUPAC rules for naming
There are about five rules that has to be followed for naming an alkane and they are,
- The longest continuous carbon chain in the compound has to be identified. This is known as parent compound. From this the parent name is obtained. Suffix “–ane” (for alkane) is added at the end of the prefix which gives information about the number of carbon atoms.
- Numbering has to be done so that the lowest number is given to the first group that is encountered in the parent chain.
- Naming and numbering has to be given for each atom or group that is attached to the parent chain. Numbering has to be done in a way that substituents get the least numbering.
- If the same substitution is present in the parent chain more than once, a separate prefix is added which tells about the number of times the substituent occurs. Prefixes used are di-, tri-, tetra-, penta- etc.
- Name of the substituents has to be placed in an alphabetical order before the parent compound name.
(b)
Interpretation:
IUPAC name has to be determined for the given compound.
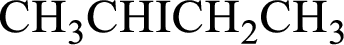
Concept Introduction:
Refer part (a).
(c)
Interpretation:
IUPAC name has to be determined for the given compound.
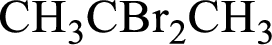
Concept Introduction:
Refer part (a).
(d)
Interpretation:
IUPAC name has to be determined for the given compound.
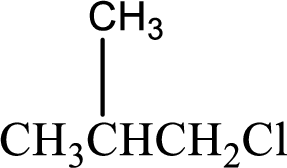
Concept Introduction:
Refer part (a).
(e)
Interpretation:
IUPAC name has to be determined for the given compound.
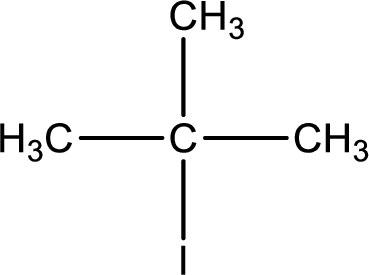
Concept Introduction:
Refer part (a).
Want to see the full answer?
Check out a sample textbook solution
Chapter 10 Solutions
GENERAL,ORGANIC,+BIOCHEMISTRY(LL)-PKG
- Please explain how to calculate the pH.arrow_forwardI'm having trouble with converting lewis diagrams into VSEPR diagrams. I currently have this example of C2BrCl3 which I want to turn into a lewis structure, but I'm not sure what steps I need to do in order to do so. I have the table written down, however, there's two central atoms so what would I do? There seems to be 4 electron domains on the carbon atom and no lone pairs so it would seem like this shape would be tetrahedral. Here's what I have now. Thanks!arrow_forwardWe discussed the solid phase resin using in peptide synthesis. Provide a mechanism, for its formation. DRAW THE MECHANISM.arrow_forward
- (a) 21.8 Name the following compounds. & (b) Br (e) O₂N. (h) H (c) Br (d) NH2 ☑N Br H ہیں Ph (g) OMe бл .0-0.e 21.9 Draw a structural formula for each compound. (a) 2,3-Dinitrotoluene (c) Diphenylmethanol (e) p-Nitroaniline (b) 3-Propylanisole (d) m-Propylphenol (f) Pentabromobenzenearrow_forwardIs this the major product of this reaction?arrow_forwardPlease helparrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





