
Concept explainers
(a)
Interpretation:
The given reaction is to be completed and explained to give the principal products.
Concept introduction:
The nucleophilic substitution reactions are the reactions in which nucleophile attacks the electrophilic centre and eliminates another group. These reactions depend upon the nucleophilicity and concentration of the nucleophile.
The
The
Answer to Problem 10.59AP
The complete reaction is shown below.
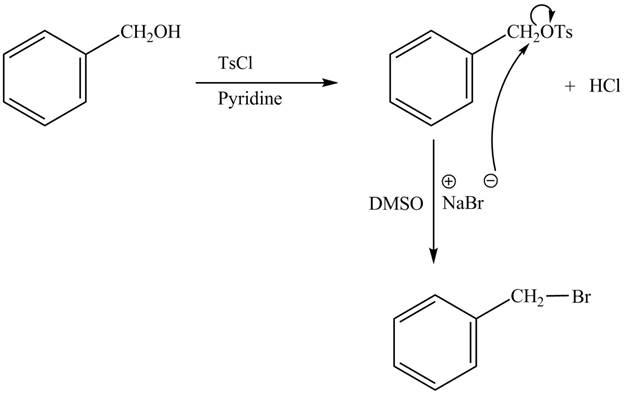
The tosyl chloride is used to make the hydroxide group a good leaving group by replacing its hydrogen with tosyl group. The
Explanation of Solution
The given reaction is shown below.
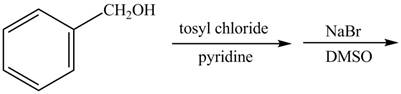
Figure 1
The complete reaction with the products is shown below.
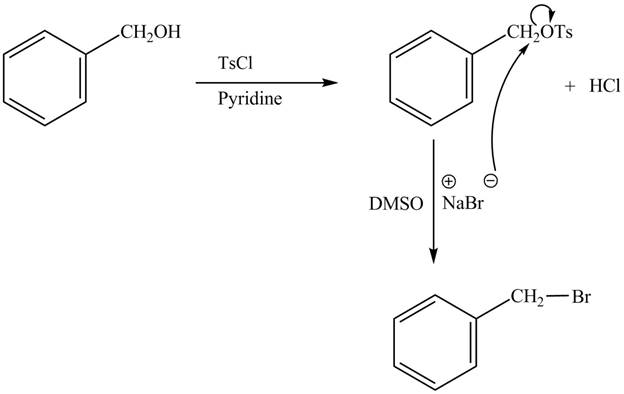
Figure 2
The reaction of the alcohols with tosyl chloride is the reaction to make the hydroxide group a good leaving group. The hydrogen is replaced by the tosyl group. The ![]() to give the halide. The product thus obtained in the end is benzyl bromide.
to give the halide. The product thus obtained in the end is benzyl bromide.
The completed reaction is shown in Figure 2.
(b)
Interpretation:
The given reaction is to be completed to give the principal products and to be explained.
Concept introduction:
The nucleophilic substitution reactions are the reactions in which nucleophile attacks the electrophilic centre and eliminates another group. These reactions depend upon the nucleophilicity and concentration of the nucleophile.
The
Answer to Problem 10.59AP
The complete reaction is shown below.
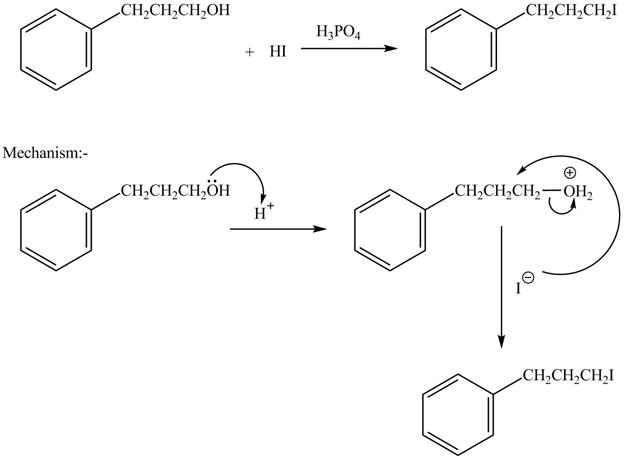
The acid is used to make the hydroxide group a good leaving group. The iodide group than substitutes the protonated hydroxide group to give halide product.
Explanation of Solution
The given reaction is shown below.
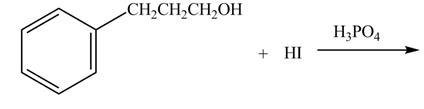
Figure 3
The complete reaction with the products is shown below.

Figure 4
The hydroxide group in alcohols is not a good leaving group in order to perform a nucleophilic substitution reaction on alcohols to produce more compounds. Hydroxide group is made a good leaving group by protonating the hydroxide group in the first step. After then the iodide ion attacks and eliminates protonated hydroxide group to halide product.
The completed reaction is shown in Figure 4.
(c)
Interpretation:
The given reaction is to be completed to give the principal products and to be explained.
Concept introduction:
The nucleophilic substitution reactions are the reactions in which nucleophile attacks the electrophilic centre and eliminates another group. These reactions depend upon the nucleophilicity and concentration of the nucleophile.
The
Answer to Problem 10.59AP
The complete reaction is shown below.
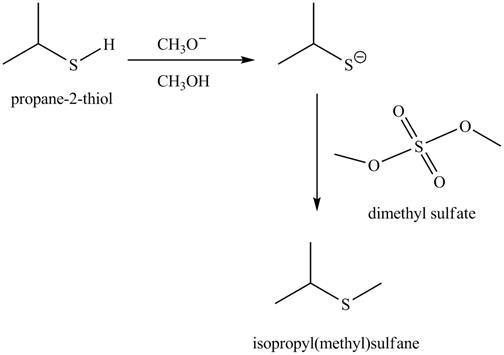
The acid-base reaction between the thiol group and methoxide ion takes place first to give sulfide ion. The sulfide ion then reacts with methylating agent dimethyl sulfate to give the methylated product isopropyl(methyl) sulfane.
Explanation of Solution
The given reaction is shown below.
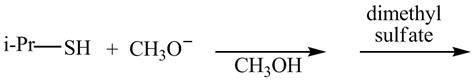
Figure 5
The complete reaction with the products is shown below.
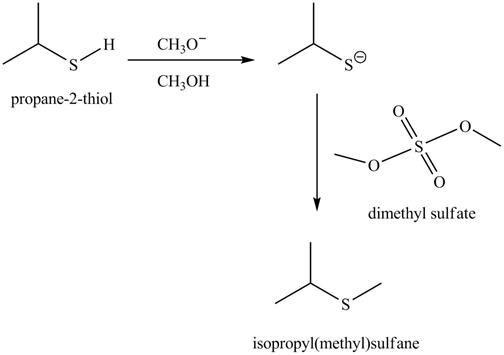
Figure 6
The methoxide ion acts as a base and takes away the hydrogen of the thiol group of
The completed reaction is shown in Figure 6.
(d)
Interpretation:
The given reaction is to be completed to give the principal products and to be explained.
Concept introduction:
The nucleophilic substitution reactions are the reactions in which nucleophile attacks the electrophilic centre and eliminates another group. These reactions depend upon the nucleophilicity and concentration of the nucleophile.
The
Answer to Problem 10.59AP
The complete reaction is shown below.
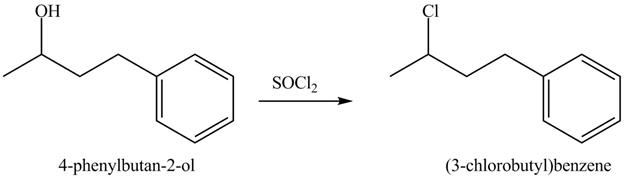
This is an
Explanation of Solution
The given reaction is shown below.
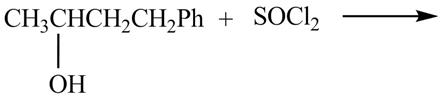
Figure 7
The complete reaction with the products is shown below.

Figure 8
The reaction of alcohols with thionyl chloride is a
The completed reaction is shown in Figure 8.
(e)
Interpretation:
The given reaction is to be completed to give the principal products and to be explained.
Concept introduction:
The nucleophilic substitution reactions are the reactions in which nucleophile attacks the electrophilic centre and eliminates another group. These reactions depend upon the nucleophilicity and concentration of the nucleophile.
The
Answer to Problem 10.59AP
The complete reaction is shown below.
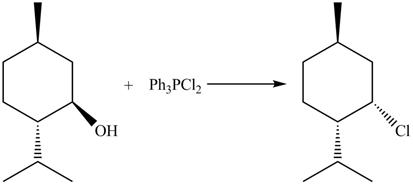
This is an
Explanation of Solution
The given reaction is shown below.
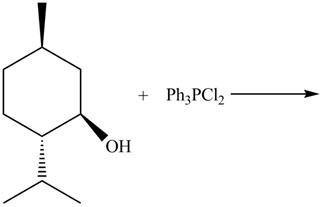
Figure 9
The complete reaction with the products is shown below.
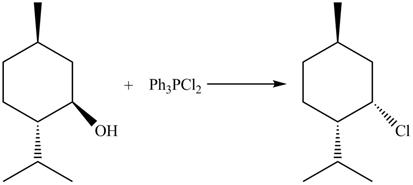
Figure 10
The reaction of alcohols with triphenylphosphine dichloride is a
The completed reaction is shown in Figure 10.
(f)
Interpretation:
The given reaction is to be completed to give the principal products and to be explained.
Concept introduction:
An
Answer to Problem 10.59AP
The complete reaction is shown below.
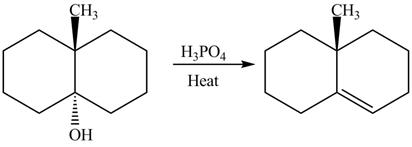
The reaction between an alcohol and acid with heating undergoes dehydration reaction to give alkene as a product.
Explanation of Solution
The given reaction is shown below.
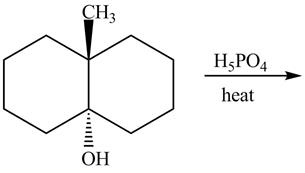
Figure 11
The complete reaction with the products is shown below.

Figure 12
The reaction of alcohols with acids and heat is an
The completed reaction is shown in Figure 12.
(g)
Interpretation:
The given reaction is to be completed to give the principal products and to be explained.
Concept introduction:
An
Answer to Problem 10.59AP
The complete reaction is shown below.
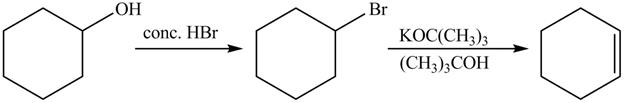
The first reaction is the nucleophilic substitution reaction of hydroxide group by the bromide ion. The second reaction is the elimination reaction in which strong base
Explanation of Solution
The given reaction is shown below.
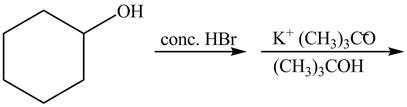
Figure 13
The complete reaction with the products is shown below.

Figure 14
The first step of the reaction is a
The completed reaction is shown in Figure 14.
(h)
Interpretation:
The given reaction is to be completed to give the principal products and to be explained.
Concept introduction:
An
Answer to Problem 10.59AP
The complete reaction is shown below.
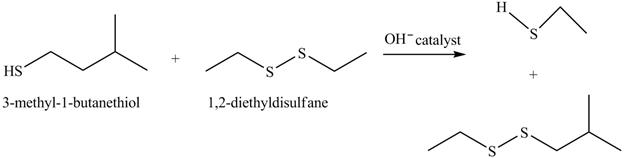
The acid-base reaction between the thiol group and hydroxide ion takes place first to give sulfide ion. The sulfide ion then reacts with diethyl sulfane to give a mixture of thiol and disulfide.
Explanation of Solution
The given reaction is shown below.
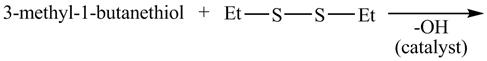
Figure 15
The complete reaction with the products is shown below.
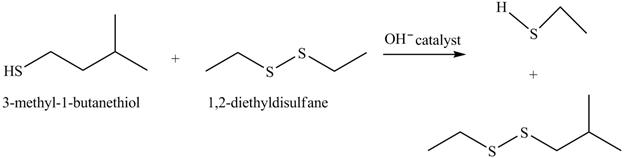
Figure 16
The hydroxide ion acts as a base and takes away the hydrogen of the thiol group of
The completed reaction is shown in Figure 16.
Want to see more full solutions like this?
Chapter 10 Solutions
ORGANIC CHEMISTRY SAPLING ACCESS + ETEX
- What is the IUPAC name of the following compound? CH₂CH₂ H CI H₂CH₂C H CH₂ Selected Answer: O (35,4R)-4 chloro-3-ethylpentane Correctarrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electrons-pushing arrows for the following reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. I I I H Select to Add Arrows HCI, CH3CH2OHarrow_forward
- Curved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and the follow the arrows to draw the intermediate and product in this reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the curved arrows to draw the intermediates and product of the following reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the arrows to draw the intermediate and the product in this reaction or mechanistic step(s).arrow_forward
- Look at the following pairs of structures carefully to identify them as representing a) completely different compounds, b) compounds that are structural isomers of each other, c) compounds that are geometric isomers of each other, d) conformers of the same compound (part of structure rotated around a single bond) or e) the same structure.arrow_forwardGiven 10.0 g of NaOH, what volume of a 0.100 M solution of H2SO4 would be required to exactly react all the NaOH?arrow_forward3.50 g of Li are combined with 3.50 g of N2. What is the maximum mass of Li3N that can be produced? 6 Li + N2 ---> 2 Li3Narrow_forward
- 3.50 g of Li are combined with 3.50 g of N2. What is the maximum mass of Li3N that can be produced? 6 Li + N2 ---> 2 Li3Narrow_forwardConcentration Trial1 Concentration of iodide solution (mA) 255.8 Concentration of thiosulfate solution (mM) 47.0 Concentration of hydrogen peroxide solution (mM) 110.1 Temperature of iodide solution ('C) 25.0 Volume of iodide solution (1) used (mL) 10.0 Volume of thiosulfate solution (5:03) used (mL) Volume of DI water used (mL) Volume of hydrogen peroxide solution (H₂O₂) used (mL) 1.0 2.5 7.5 Time (s) 16.9 Dark blue Observations Initial concentration of iodide in reaction (mA) Initial concentration of thiosulfate in reaction (mA) Initial concentration of hydrogen peroxide in reaction (mA) Initial Rate (mA's)arrow_forwardDraw the condensed or line-angle structure for an alkene with the formula C5H10. Note: Avoid selecting cis-/trans- isomers in this exercise. Draw two additional condensed or line-angle structures for alkenes with the formula C5H10. Record the name of the isomers in Data Table 1. Repeat steps for 2 cyclic isomers of C5H10arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





