
Concept explainers
(a)
Interpretation:
The increasing order of acidity of the alcohols is to be stated.
Concept introduction:
Acidity of a molecule is dependent on the value of
Answer to Problem 10.46AP
The increasing order of acidity of the alcohols is given below.
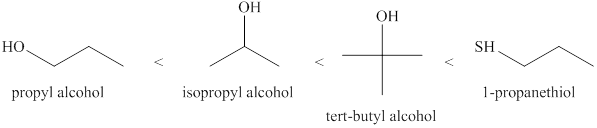
Explanation of Solution
Acidity of the molecules is dependent upon the stability of the ion formed after releasing hydrogen ion.
Sulfur atom present in
Order of the acidity is shown below in Figure 1.

Figure 1
The increasing order of acidity of the molecules is
(b)
Interpretation:
The increasing order of acidity of the molecules is to be stated.
Concept introduction:
Acidity of a molecule is dependent on the value of
Answer to Problem 10.46AP
The increasing order of acidity of the molecules is given below.
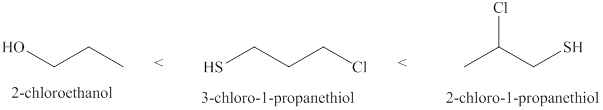
Explanation of Solution
Acidity of the molecules is dependent upon the stability of the ion formed after releasing hydrogen ion. Sulfur atom present in
Therefore, increasing order of the given molecules is shown below in Figure 2.
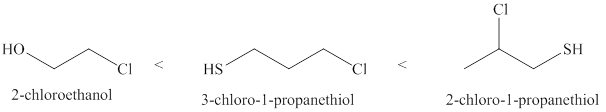
Figure 2
The increasing order of acidity of the given molecules is
(c)
Interpretation:
The increasing order of acidity of the molecules is to be stated.
Concept introduction:
Acidity of a molecule is dependent on the value of
Answer to Problem 10.46AP
The increasing order of acidity of the molecules is stated below.
Explanation of Solution
Acidity of the molecules is dependent upon the stability of the ion formed after releasing hydrogen ion. Nitrogen atom which is positively charged, stabilizes the negative charge which is generated after releasing the hydrogen ion. Therefore, structure 3 is the most stable structure with most acidic character. Position of the electronegative atom also determines the strength of an acid. Closer the electronegative atom to the generated negative charge after releasing the hydrogen ion, more is the strength of the acid.
Therefore, increasing order of the given molecules is stated below.
The increasing order of acidity of the given molecules is
(d)
Interpretation:
The increasing order of acidity of the molecules is to be stated.
Concept introduction:
Acidity of a molecule is dependent on the value of
Answer to Problem 10.46AP
The increasing order of acidity of the molecules is stated below.
Explanation of Solution
Acidity of the molecules is dependent upon the stability of the ion formed after releasing the hydrogen ion. In structure
Therefore, increasing order of acidity of the given molecules is stated below.
The increasing order of acidity of the given molecules is
(e)
Interpretation:
The increasing order of acidity of the molecules is to be stated.
Concept introduction:
Acidity of a molecule is dependent on the value of
Answer to Problem 10.46AP
The increasing order of acidity of the molecules is stated below.
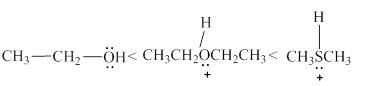
Explanation of Solution
Acidity of the molecules is dependent upon the stability of the ion formed after releasing the hydrogen ion. In structure
Therefore, increasing order of the given molecules is stated below in Figure 3.
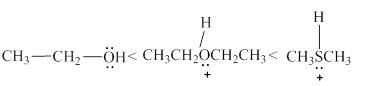
Figure 3
The increasing order of acidity of the given molecules is shown above in Figure 3.
Want to see more full solutions like this?
Chapter 10 Solutions
ORGANIC CHEMISTRY SAPLING ACCESS + ETEX
- H-Br Energy 1) Draw the step-by-step mechanism by which 3-methylbut-1-ene is converted into 2-bromo-2-methylbutane. 2) Sketch a reaction coordinate diagram that shows how the internal energy (Y- axis) of the reacting species change from reactants to intermediate(s) to product. Brarrow_forward2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). C5H10 H-CI CH2Cl2 CIarrow_forwardDraw the products of the stronger acid protonating the other reactant. དའི་སྐད”“ H3C OH H3C CH CH3 KEq Product acid Product basearrow_forward
- Draw the products of the stronger acid protonating the other reactant. H3C NH2 NH2 KEq H3C-CH₂ 1. Product acid Product basearrow_forwardWhat alkene or alkyne yields the following products after oxidative cleavage with ozone? Click the "draw structure" button to launch the drawing utility. draw structure ... andarrow_forwardDraw the products of the stronger acid protonating the other reactant. H3C-C=C-4 NH2 KEq CH H3C `CH3 Product acid Product basearrow_forward
- 2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). C5H10 Br H-Br CH2Cl2 + enant.arrow_forwardDraw the products of the stronger acid protonating the other reactant. KEq H₂C-O-H H3C OH Product acid Product basearrow_forwardDraw the products of the stronger acid protonating the other reactant. OH KEq CH H3C H3C `CH3 Product acid Product basearrow_forward
- 2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). Ph H-I CH2Cl2arrow_forward3 attempts left Check my work Draw the products formed in the following oxidative cleavage. [1] 03 [2] H₂O draw structure ... lower mass product draw structure ... higher mass productarrow_forward2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). H-Br CH2Cl2arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


