
Concept explainers
(a)
Interpretation:
The given reaction is to be completed and explained to give the principal products.
Concept introduction:
The nucleophilic substitution reactions are the reactions in which nucleophile attacks the electrophilic centre and eliminates another group. These reactions depend upon the nucleophilicity and concentration of the nucleophile.
The
The
Answer to Problem 10.59AP
The complete reaction is shown below.
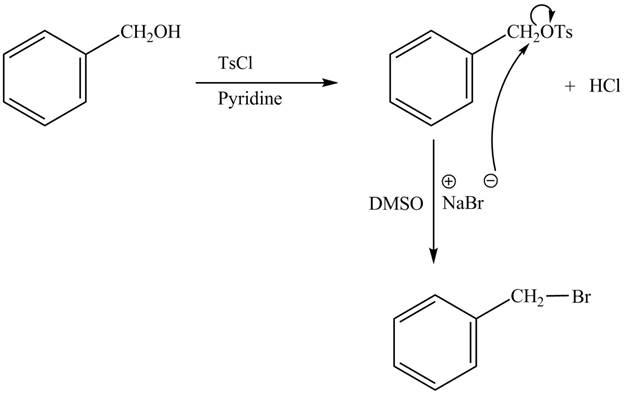
The tosyl chloride is used to make the hydroxide group a good leaving group by replacing its hydrogen with tosyl group. The
Explanation of Solution
The given reaction is shown below.
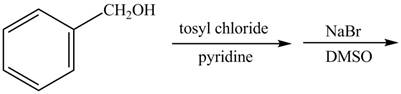
Figure 1
The complete reaction with the products is shown below.
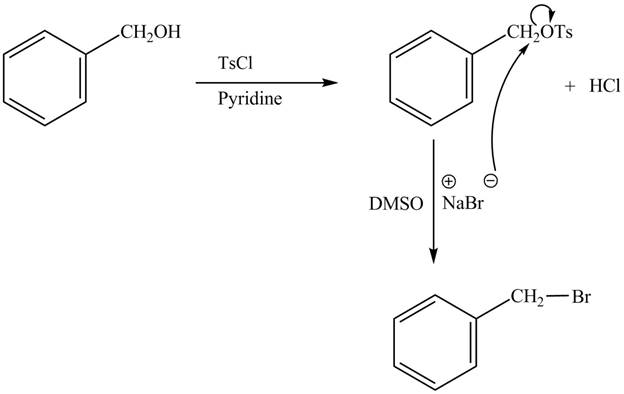
Figure 2
The reaction of the alcohols with tosyl chloride is the reaction to make the hydroxide group a good leaving group. The hydrogen is replaced by the tosyl group. The ![]() to give the halide. The product thus obtained in the end is benzyl bromide.
to give the halide. The product thus obtained in the end is benzyl bromide.
The completed reaction is shown in Figure 2.
(b)
Interpretation:
The given reaction is to be completed to give the principal products and to be explained.
Concept introduction:
The nucleophilic substitution reactions are the reactions in which nucleophile attacks the electrophilic centre and eliminates another group. These reactions depend upon the nucleophilicity and concentration of the nucleophile.
The
Answer to Problem 10.59AP
The complete reaction is shown below.
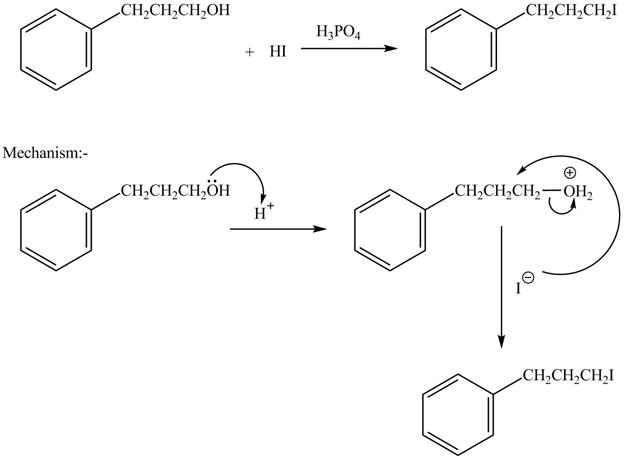
The acid is used to make the hydroxide group a good leaving group. The iodide group than substitutes the protonated hydroxide group to give halide product.
Explanation of Solution
The given reaction is shown below.
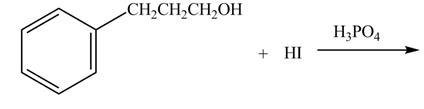
Figure 3
The complete reaction with the products is shown below.

Figure 4
The hydroxide group in alcohols is not a good leaving group in order to perform a nucleophilic substitution reaction on alcohols to produce more compounds. Hydroxide group is made a good leaving group by protonating the hydroxide group in the first step. After then the iodide ion attacks and eliminates protonated hydroxide group to halide product.
The completed reaction is shown in Figure 4.
(c)
Interpretation:
The given reaction is to be completed to give the principal products and to be explained.
Concept introduction:
The nucleophilic substitution reactions are the reactions in which nucleophile attacks the electrophilic centre and eliminates another group. These reactions depend upon the nucleophilicity and concentration of the nucleophile.
The
Answer to Problem 10.59AP
The complete reaction is shown below.
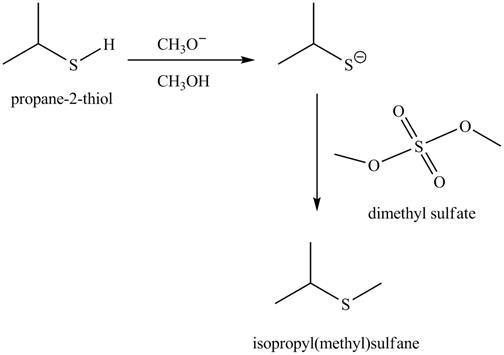
The acid-base reaction between the thiol group and methoxide ion takes place first to give sulfide ion. The sulfide ion then reacts with methylating agent dimethyl sulfate to give the methylated product isopropyl(methyl) sulfane.
Explanation of Solution
The given reaction is shown below.
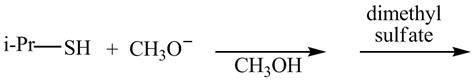
Figure 5
The complete reaction with the products is shown below.
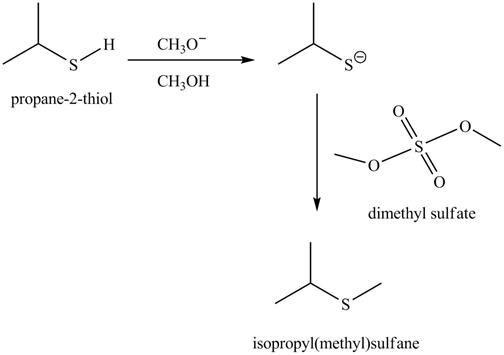
Figure 6
The methoxide ion acts as a base and takes away the hydrogen of the thiol group of
The completed reaction is shown in Figure 6.
(d)
Interpretation:
The given reaction is to be completed to give the principal products and to be explained.
Concept introduction:
The nucleophilic substitution reactions are the reactions in which nucleophile attacks the electrophilic centre and eliminates another group. These reactions depend upon the nucleophilicity and concentration of the nucleophile.
The
Answer to Problem 10.59AP
The complete reaction is shown below.
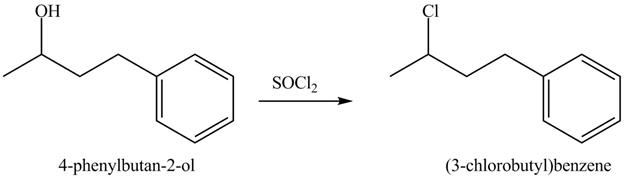
This is an
Explanation of Solution
The given reaction is shown below.
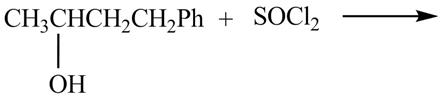
Figure 7
The complete reaction with the products is shown below.

Figure 8
The reaction of alcohols with thionyl chloride is a
The completed reaction is shown in Figure 8.
(e)
Interpretation:
The given reaction is to be completed to give the principal products and to be explained.
Concept introduction:
The nucleophilic substitution reactions are the reactions in which nucleophile attacks the electrophilic centre and eliminates another group. These reactions depend upon the nucleophilicity and concentration of the nucleophile.
The
Answer to Problem 10.59AP
The complete reaction is shown below.
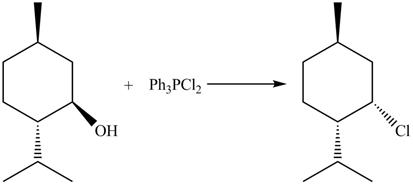
This is an
Explanation of Solution
The given reaction is shown below.
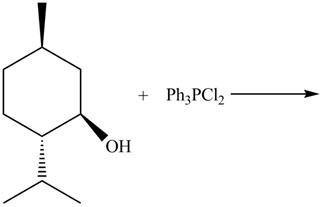
Figure 9
The complete reaction with the products is shown below.
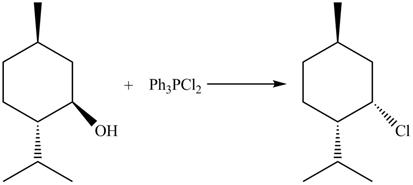
Figure 10
The reaction of alcohols with triphenylphosphine dichloride is a
The completed reaction is shown in Figure 10.
(f)
Interpretation:
The given reaction is to be completed to give the principal products and to be explained.
Concept introduction:
An
Answer to Problem 10.59AP
The complete reaction is shown below.
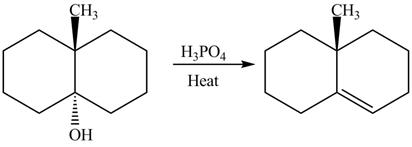
The reaction between an alcohol and acid with heating undergoes dehydration reaction to give alkene as a product.
Explanation of Solution
The given reaction is shown below.
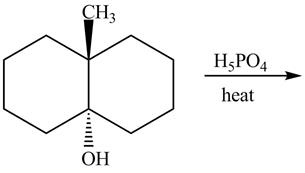
Figure 11
The complete reaction with the products is shown below.

Figure 12
The reaction of alcohols with acids and heat is an
The completed reaction is shown in Figure 12.
(g)
Interpretation:
The given reaction is to be completed to give the principal products and to be explained.
Concept introduction:
An
Answer to Problem 10.59AP
The complete reaction is shown below.
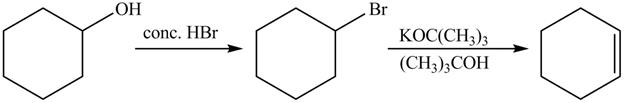
The first reaction is the nucleophilic substitution reaction of hydroxide group by the bromide ion. The second reaction is the elimination reaction in which strong base
Explanation of Solution
The given reaction is shown below.
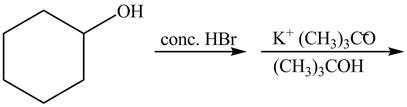
Figure 13
The complete reaction with the products is shown below.

Figure 14
The first step of the reaction is a
The completed reaction is shown in Figure 14.
(h)
Interpretation:
The given reaction is to be completed to give the principal products and to be explained.
Concept introduction:
An
Answer to Problem 10.59AP
The complete reaction is shown below.
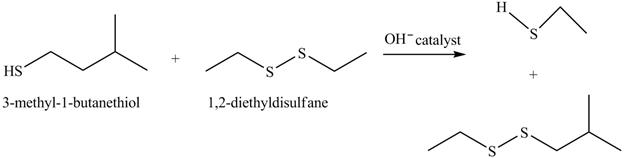
The acid-base reaction between the thiol group and hydroxide ion takes place first to give sulfide ion. The sulfide ion then reacts with diethyl sulfane to give a mixture of thiol and disulfide.
Explanation of Solution
The given reaction is shown below.
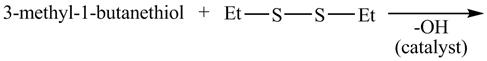
Figure 15
The complete reaction with the products is shown below.
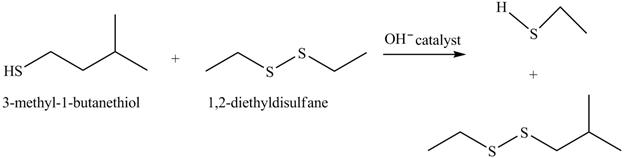
Figure 16
The hydroxide ion acts as a base and takes away the hydrogen of the thiol group of
The completed reaction is shown in Figure 16.
Want to see more full solutions like this?
Chapter 10 Solutions
ORGANIC CHEM +SG +SAPLING >IP<
- Draw the structure of serine at pH 6.8. Click and drag to start drawing a structure. : d كarrow_forwardTake a look at this molecule, and then answer the questions in the table below it. CH2OH H H H OH OH OH CH2OH H H H H OH H H OH H OH Is this a reducing sugar? yes α β ロ→ロ no ☑ yes Does this molecule contain a glycosidic bond? If you said this molecule does contain a glycosidic bond, write the symbol describing it. O no 0+0 If you said this molecule does contain a glycosidic bond, write the common names (including anomer and enantiomer labels) of the molecules that would be released if that bond were hydrolyzed. If there's more than one molecule, separate each name with a comma. ☐arrow_forwardAnswer the questions in the table below about this molecule: H₂N-CH₂ -C—NH–CH–C—NH–CH—COO- CH3 CH CH3 What kind of molecule is this? 0= CH2 C If you said the molecule is a peptide, write a description of it using 3-letter codes separated ☐ by dashes. polysaccharide peptide amino acid phospolipid none of the above Хarrow_forward
- Draw a Haworth projection of a common cyclic form of this monosaccharide: CH₂OH C=O HO H H -OH H OH CH₂OH Click and drag to start drawing a structure. : ☐ Х S '☐arrow_forwardNucleophilic Aromatic Substitution 22.30 Predict all possible products formed from the following nucleophilic substitution reactions. (a) (b) 9 1. NaOH 2. HCI, H₂O CI NH₁(!) +NaNH, -33°C 1. NaOH 2. HCl, H₂Oarrow_forwardSyntheses 22.35 Show how to convert toluene to these compounds. (a) -CH,Br (b) Br- -CH3 22.36 Show how to prepare each compound from 1-phenyl-1-propanone. 1-Phenyl-1-propanone ہتی. Br. (b) Br (racemic) 22.37 Show how to convert ethyl benzene to (a) 2,5-dichlorobenzoic acid and (b) 2,4-dichlorobenzoic acid. 22.38 Show reagents and conditions to bring about the following conversions. (a) 9 NH2 8 CO₂H NH2 CO₂Et (d) NO2 NH2 S NH₂ NO2 CHS CHarrow_forward
- ive the major organic product(s) of each of the following reactions or sequences of reactions. Show all rant stereochemistry. [10 only] A. B. NaN3 1. LiAlH4, ether Br 2. H₂O CH3 HNO3 H₂/Pt H₂SO ethanol C. 0 0 CH3CC1 NaOH NHCCH AICI H₂O . NH₂ CH3CH2 N CH2CH3 + HCI CH₂CH 3 1. LIAIH, THE 2. H₂Oarrow_forwardCalculate the stoichiometric amount of CaCl2 needed to convert all of the CuSO4 into CuCl2.arrow_forwardH CH تنی Cl 1. NaCN, DMF 2. LIAIH4, ether H₂O pyridine N NH₂ 5 CH H 1 HNO, H₂SO 2. Nal NH2 Br Br HNO₂ CuCl H₂SO HCI CH3 H3C NN HSO KCN CuCN 1. HNO₂, H₂SO O₂N NH2 2. OH ཀ་ལས། །ས་ཅན་ :i་དེ་མ་མ་སེ་ NH₂ CH3 1. HNO₂, H₂SO4 2. H3PO₂ 1 HNO2, H2SO4 2. Nalarrow_forward
- ive the major organic product(s) of each of the following reactions or sequences of reactions. Show all rant stereochemistry. [10 only] A. B. NaN3 1. LiAlH4, ether Br 2. H₂O CH3 HNO3 H₂/Pt H₂SO ethanol C. 0 0 CH3CC1 NaOH NHCCH AICI H₂O . NH₂ CH3CH2 N CH2CH3 + HCI CH₂CH 3 1. LIAIH, THE 2. H₂Oarrow_forwardIf a pharmacy chain sold 65 million 500-mg tablets of aspirin, how many US tons of aspirin does this represent? Report your answer to 2 significant figures.arrow_forwardHere are the options: reducing a monosaccharide a non reducing disaccharide amylopectin cellulose 1,4' beta- glycosidearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





