
International Edition---engineering Mechanics: Statics 4th Edition
4th Edition
ISBN: 9781305856240
Author: Pytel
Publisher: Cengage
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10, Problem 10.57P
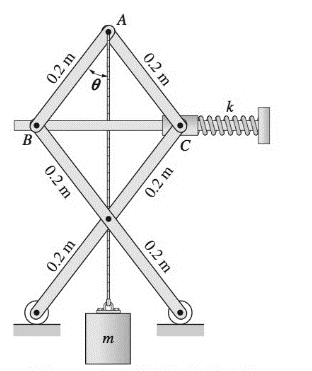
Find the stable equilibrium position of the system described in Prob. 10.56 if m = 2.06 kg.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
4. Determine which of the following flow fields represent a possible
incompressible flow?
(a) u= x²+2y+z; v=x-2y+z;w= -2xy + y² + 2z
a
(b) V=U cose
U coso 1 (9)
[1-9]
Usino |1 (4)]
[+]
V=-Usin 1+1
3. Determine the flow rate through the pipe line show in the figure in ft³/s,
and determine the pressures at A and C, in psi.
5'
B
C
12°
20'
D
6"d
2nd-
Water
A
5. A flow is field given by V = x²₁³+xy, and determine
3
·y³j-
(a) Whether this is a one, two- or three-dimensional flow
(b) Whether it is a possible incompressible flow
(c) Determine the acceleration of a fluid particle at the location (X,Y,Z)=(1,2,3)
(d) Whether the flow is rotational or irrotational flow?
Chapter 10 Solutions
International Edition---engineering Mechanics: Statics 4th Edition
Ch. 10 - Determine the number of DOF for each of the...Ch. 10 - The uniform bar of weight W is held in equilibrium...Ch. 10 - Bars AB and AC of the mechanism are homogenous...Ch. 10 - The weight of each homogeneous bar of the linkage...Ch. 10 - The 1800-kg boat is suspended from two parallel...Ch. 10 - The 2.4-kg lamp, with center of gravity located at...Ch. 10 - The linkage is made of two homogenous bars of...Ch. 10 - For the frame shown, find the horizontal component...Ch. 10 - The four-bar linkage supports the homogeneous box...Ch. 10 - Prob. 10.10P
Ch. 10 - Determine the ratio P/Q of the forces that are...Ch. 10 - Find the vertical force P that will hold the...Ch. 10 - The linkage of the braking system consists of the...Ch. 10 - The automatic drilling robot must sustain a thrust...Ch. 10 - Determine the couple C for which the mechanism...Ch. 10 - The scissors jack is used to elevate the weight W....Ch. 10 - Prob. 10.17PCh. 10 - Calculate the torque C0 that must be applied to...Ch. 10 - Determine the force F and the angle a required to...Ch. 10 - Locate the instant center of rotation of bar AB...Ch. 10 - Prob. 10.21PCh. 10 - Determine the force P that will keep the mechanism...Ch. 10 - Prob. 10.23PCh. 10 - Prob. 10.24PCh. 10 - Prob. 10.25PCh. 10 - Determine the ratio P/Q for which the linkage will...Ch. 10 - Prob. 10.27PCh. 10 - Prob. 10.28PCh. 10 - If the input force to the compound lever is P = 30...Ch. 10 - Determine the roller reaction at F due to the...Ch. 10 - Prob. 10.31PCh. 10 - Prob. 10.32PCh. 10 - Prob. 10.33PCh. 10 - Prob. 10.34PCh. 10 - Prob. 10.35PCh. 10 - For the pliers shown, determine the relationship...Ch. 10 - When activated by the force P, the gripper cm a...Ch. 10 - Prob. 10.38PCh. 10 - The hinge is of the type used on some automobiles,...Ch. 10 - The spring attached to the sliding collar is...Ch. 10 - The weight W is suspended from end B of the...Ch. 10 - The uniform bar of weight W and length L = 1.8R...Ch. 10 - A slender homogeneous bar is bent into a right...Ch. 10 - The body shown is a composite of a hemisphere and...Ch. 10 - Prob. 10.45PCh. 10 - The uniform bar AB of weight W and length L is...Ch. 10 - Uniform rods of weights W1 and W2 are welded to...Ch. 10 - Prob. 10.48PCh. 10 - The semi-cylinder of radius r is placed on a...Ch. 10 - Prob. 10.50PCh. 10 - The spring attached to the homogenous bar of...Ch. 10 - The spring is connected to a rope that passes over...Ch. 10 - Find the equilibrium positions of the 30-lb...Ch. 10 - The mechanism of negligible weight supports the...Ch. 10 - Solve Prob. 10.54 assuming that A and B are...Ch. 10 - The stiffness of the ideal spring that is...Ch. 10 - Find the stable equilibrium position of the system...Ch. 10 - The uniform bar AB of weight W = kL is in...Ch. 10 - The weight of the uniform bar AB is W. The...Ch. 10 - The weightless bars AB and CE, together with the...Ch. 10 - Prob. 10.61PCh. 10 - The bar ABC is supported by three identical, ideal...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Solve this problem and show all of the workarrow_forwardSolve this problem and show all of the workarrow_forwarddraw the pneumatic circuit to operate a double-acting cylinder with: 1. Extension: Any of two manual conditions plus cylinder fully retracted, → Extension has both meter-in and meter-out, 2. Retraction: one manual conditions plus cylinder fully extended, → Retraction is very fast using quick exhaust valve.arrow_forward
- Correct answer is written below. Detailed and complete solution with fbd only. I will upvote, thank you. Expert solution plsarrow_forwardCorrect answer is written below. Detailed and complete solution with fbd only. I will upvote, thank you.arrow_forwardCorrect answer is written below. Detailed and complete solution with fbd only. I will upvote, thank you.arrow_forward
- Correct answer is written below. Detailed and complete solution only with fbd. I will upvote, thank you.arrow_forwardCorrect answer is written below. Detailed and complete solution only. I will upvote, thank you.arrow_forwardCorrect answer is written below. Detailed and complete solution with fbd only. I will upvote, thank you.arrow_forward
- Correct answer is written below. Detailed and complete solution only. I will upvote, thank you.arrow_forwardCorrect answer is written below. Detailed and complete solution with fbd only. I will upvote, thank you.arrow_forwardCorrect answer is written below. Detailed and complete solution only. I will upvote, thank you.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 International Edition---engineering Mechanics: St...Mechanical EngineeringISBN:9781305501607Author:Andrew Pytel And Jaan KiusalaasPublisher:CENGAGE L
International Edition---engineering Mechanics: St...Mechanical EngineeringISBN:9781305501607Author:Andrew Pytel And Jaan KiusalaasPublisher:CENGAGE L

International Edition---engineering Mechanics: St...
Mechanical Engineering
ISBN:9781305501607
Author:Andrew Pytel And Jaan Kiusalaas
Publisher:CENGAGE L
Chemical and Phase Equilibrium; Author: LearnChemE;https://www.youtube.com/watch?v=SWhZkU7e8yw;License: Standard Youtube License