
Principles of General, Organic, Biological Chemistry
2nd Edition
ISBN: 9780073511191
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10, Problem 10.38UKC
Procaine (trade name Novocain) is a local anesthetic once commonly used in medicine and dentistry.
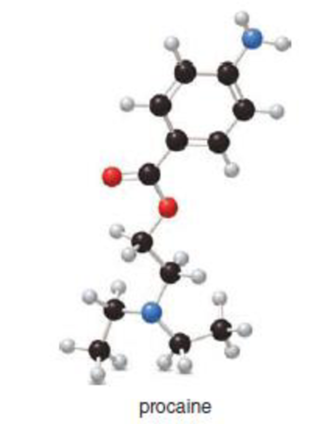
- a. What is the molecular formula of procaine?
- b. Draw in all the lone pairs on heteroatoms using a skeletal structure.
- c. Identify the
functional groups.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
a)
b)
c)
H
NaOH
heat, dehydration
+
KOH
heat, dehydration
NaOH
+
(CH3)3CCHO
heat, dehydration
Ph
show mechanism
Please draw by hand
Chapter 10 Solutions
Principles of General, Organic, Biological Chemistry
Ch. 10.1 - Prob. 10.1PCh. 10.2 - Fill in all Hs and lone pairs in each compound. a....Ch. 10.3 - Convert each compound to a condensed formula.Ch. 10.3 - Convert each condensed formula to a complete...Ch. 10.3 - Convert each skeletal structure to a complete...Ch. 10.3 - How many Hs are bonded to each indicated carbon...Ch. 10.4 - Identify the functional groups in each compound....Ch. 10.4 - For each compound: [1] Identify the functional...Ch. 10.4 - Prob. 10.9PCh. 10.4 - Prob. 10.10P
Ch. 10.4 - Convert the ball-and-stick model of the local...Ch. 10.5 - How many hydrogen atoms are present in each...Ch. 10.5 - Prob. 10.13PCh. 10.5 - Prob. 10.14PCh. 10.5 - Prob. 10.15PCh. 10.5 - Prob. 10.16PCh. 10.6 - Give the IUPAC name for each compound.Ch. 10.6 - Prob. 10.18PCh. 10.6 - Prob. 10.19PCh. 10.6 - Prob. 10.20PCh. 10.7 - Prob. 10.21PCh. 10.7 - Prob. 10.22PCh. 10.9 - Answer the following questions about pentane...Ch. 10.9 - Prob. 10.24PCh. 10.9 - Prob. 10.25PCh. 10.10 - Prob. 10.26PCh. 10 - Prob. 10.27UKCCh. 10 - Prob. 10.28UKCCh. 10 - Prob. 10.29UKCCh. 10 - Prob. 10.30UKCCh. 10 - Prob. 10.31UKCCh. 10 - The largest known cycloalkane with a single ring...Ch. 10 - Draw three constitutional isomers having molecular...Ch. 10 - Draw four constitutional isomers having molecular...Ch. 10 - Answer the following questions about the alkane...Ch. 10 - Answer the questions in Problem 10.35 for the...Ch. 10 - Prob. 10.37UKCCh. 10 - Procaine (trade name Novocain) is a local...Ch. 10 - Prob. 10.39APCh. 10 - Prob. 10.40APCh. 10 - Complete each structure by filling in all Hs and...Ch. 10 - Prob. 10.42APCh. 10 - Prob. 10.43APCh. 10 - Prob. 10.44APCh. 10 - Prob. 10.45APCh. 10 - Prob. 10.46APCh. 10 - Convert each compound to a condensed structure.Ch. 10 - Prob. 10.48APCh. 10 - Prob. 10.49APCh. 10 - Prob. 10.50APCh. 10 - Prob. 10.51APCh. 10 - Prob. 10.52APCh. 10 - Albuterol (trade names: Proventil and Ventolin) is...Ch. 10 - Prob. 10.54APCh. 10 - Prob. 10.55APCh. 10 - Prob. 10.56APCh. 10 - Prob. 10.57APCh. 10 - Prob. 10.58APCh. 10 - Prob. 10.59APCh. 10 - Prob. 10.60APCh. 10 - Prob. 10.61APCh. 10 - Prob. 10.62APCh. 10 - Prob. 10.63APCh. 10 - Prob. 10.64APCh. 10 - Prob. 10.65APCh. 10 - Prob. 10.66APCh. 10 - Prob. 10.67APCh. 10 - Prob. 10.68APCh. 10 - Prob. 10.69APCh. 10 - Prob. 10.70APCh. 10 - Give the IUPAC name for each compound.Ch. 10 - Prob. 10.72APCh. 10 - Prob. 10.73APCh. 10 - Prob. 10.74APCh. 10 - Prob. 10.75APCh. 10 - Prob. 10.76APCh. 10 - Give the structure corresponding to each IUPAC...Ch. 10 - Prob. 10.78APCh. 10 - Prob. 10.79APCh. 10 - Each of the following IUPAC names is incorrect....Ch. 10 - Prob. 10.81APCh. 10 - Prob. 10.82APCh. 10 - Prob. 10.83APCh. 10 - Prob. 10.84APCh. 10 - Prob. 10.85APCh. 10 - Prob. 10.86APCh. 10 - Write a balanced equation for the incomplete...Ch. 10 - Prob. 10.88APCh. 10 - Prob. 10.89APCh. 10 - Prob. 10.90APCh. 10 - Prob. 10.91APCh. 10 - Prob. 10.92APCh. 10 - Answer the following questions for the cycloalkane...Ch. 10 - Prob. 10.94APCh. 10 - Prob. 10.95CPCh. 10 - Prob. 10.96CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 3. Predict the major product and give a mechanism for the following reactions: (CH3)3COH/H₂SO4 a) b) NC CH₂O c) LOCH, (CH3)3COH/H2SO4 H,SO -OHarrow_forwardIndicate if the aldehyde shown reacts with the provided nucleophiles in acid or base conditions. a NaBH4 be Li eli -NH2 P(Ph3) f KCN g OH excess h CH3OH i NaCHCCH3arrow_forwardPredict the major products of the following organic reaction: + A ? Some important notes: • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. Explanation Check Click and drag to start drawing a structure. C © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centearrow_forward
- Polar solutes are most likely to dissolve into _____, and _____ are most likely to dissolve into nonpolar solvents. A. nonpolar solutes; polar solvents B. nonpolar solvents; polar solvents C. polar solvents; nonpolar solutes D. polar solutes; nonpolar solventsarrow_forwardDeducing the Peactants Can the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? ? Δ If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. If your answer is no, check the box under the drawing area instead. Explanation Check Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Xarrow_forwardDraw all 8 stereoisomers, circling each pair of enantiomer(s)/ mirror image compound(s)arrow_forward
- Bookmarks Profiles Tab Window Help Chemical Formula - Aktiv Che X + → C 11 a app.aktiv.com Google Chrome isn't your default browser Set as default Question 12 of 16 Q Fri Feb 2 Verify it's you New Chrome availabl- Write the balanced molecular chemical equation for the reaction in aqueous solution for mercury(I) nitrate and chromium(VI) sulfate. If no reaction occurs, simply write only NR. Be sure to include the proper phases for all species within the reaction. 3 Hg(NO3)2(aq) + Cг2(SO4)3(aq) → 3 Hg₂SO (s) + 2 Cr(NO3), (aq) ean Ui mate co ence an climate bility inc ulnerabili women, main critic CLIMATE-INI ernational + 10 O 2 W FEB 1 + 4- 3- 2- 2 2 ( 3 4 NS 28 2 ty 56 + 2+ 3+ 4+ 7 8 9 0 5 (s) (1) Ch O 8 9 (g) (aq) Hg NR CI Cr x H₂O A 80 Q A DII A F2 F3 FA F5 F6 F7 F8 F9 #3 EA $ do 50 % 6 CO & 7 E R T Y U 8 ( 9 0 F10 34 F11 川 F12 Subr + delete 0 { P }arrow_forwardDeducing the reactants of a Diels-Alder reaction n the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? ? Δ • If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. • If your answer is no, check the box under the drawing area instead. Explanation Check Click and drag to start drawing a structure. >arrow_forwardPredict the major products of the following organic reaction: + Some important notes: A ? • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. Explanation Check Click and drag to start drawing a structure.arrow_forward
- if the answer is no reaction than state that and please hand draw!arrow_forward"I have written solutions in text form, but I need experts to rewrite them in handwriting from A to Z, exactly as I have written, without any changes."arrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY