
Concept explainers
Give the structure corresponding to each IUPAC name.
- a. 3-ethylhexane
- b. 3-ethyl-3-methyloctane
- c. 2,3,4,5-tetramethyldecane
- d. cyclononane
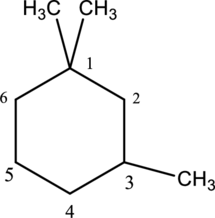
- e. 1,1,3-trimethylcyclohexane
a.
Interpretation:
The structure of given IUPAC name 3-ethylhexane has to be drawn.
Concept Introduction:
IUPAC Nomenclature:
The system which is used to name an organic compound is known as IUPAC Nomenclature. IUPAC are known as International Union of Pure and Applied Chemistry.
There are some rules followed for writing IUPAC Nomenclature for alkane compound is given below,
- The longest chain in the compound is known as parent name which represent the number of carbon atom present in the continuous carbon chain. The root name for alkane compound is –ane. The single bonds are formed in alkane compounds.
- The suffix group denotes the functional group present in a molecule. The prefix group indicates the identity, location and number of substituents attached to the carbon compound.
- Then name and number the substituents present in compounds. Then use prefix di-to represent two groups, tri- refers to three groups and so on. Then numbers has to be separated by using commas and the letters from numbers has to be separated by using dashes. Then numbers has to be separated by using commas and the letters from numbers has to be separated by using dashes.
There are some rules followed for writing IUPAC Nomenclature for cycloalkane compounds:
- First identify the number of carbon atoms present in the ring. The numbers of carbon atoms present in ring is used as the parent name. Add suffix name -ane and prefix name cyclo- to the parent name of cycloalkane compound.
- The suffix group denotes the functional group present in a molecule. Then number the substituents present in compound. If there is a single substituent no need to mention number. If the ring has more than one substituent, then start numbering the ring with lowest number to the substituents in alphabetical order. Hence combine the parts to form IUPAC name.
Explanation of Solution
The structure of given IUPAC name 3-ethylhexane has to be drawn. The longest continuous chain has six carbon atoms and so the parent name is hex-. The root name of alkane compounds are –ane has to be added as suffix name to the parent name. Hence the parent name is given as hexane.
The carbon skeleton for hexane is given below,
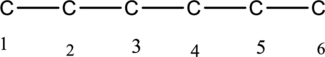
Then number and name the substituent in alphabetical order. The substituent ethyl group
The substituent linked to carbon chain is given below,
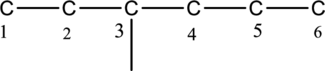
The structure of given IUPAC name 3-ethylhexane is drawn below,
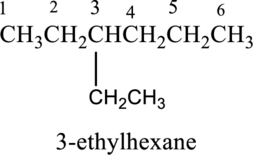
b.
Interpretation
The structure of given IUPAC name 3-ethyl-3-methyloctane has to be drawn.
Concept Introduction:
Refer part a.
Explanation of Solution
The structure of given IUPAC name 3-ethyl-3-methyloctane has to be drawn. The longest continuous chain has eight carbon atoms and so the parent name is oct-. The root name of alkane compounds are –ane has to be added as suffix name to the parent name. Hence the parent name is given as octane.
The carbon skeleton for octane is given below,
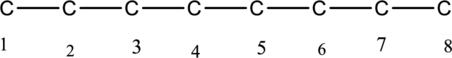
Then number and name the substituents in alphabetical order. The substituent ethyl group
The substituents linked to carbon chain is drawn below,
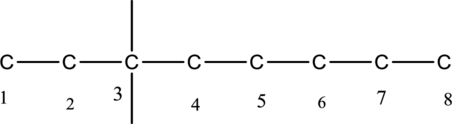
The structure of given IUPAC name 3-ethyl-3-methyloctane is drawn below,
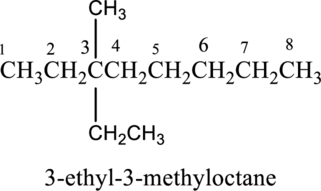
c.
Interpretation
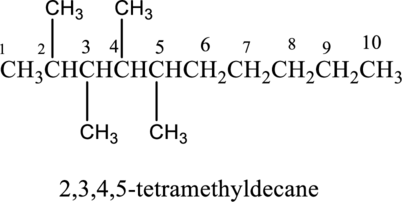
The structure of given IUPAC name 2,3,4,5, -tetramethyldecane has to be drawn.
Concept Introduction:
Refer part a.
Explanation of Solution
The structure of given IUPAC name 2,3,4,5-tetramethyldecane has to be drawn. The longest continuous chain has ten carbon atoms and so the parent name is dec-. The root name of alkane compounds are –ane has to be added as suffix name to the parent name. Hence the parent name is given as decane.
The carbon skeleton for decane is drawn below,
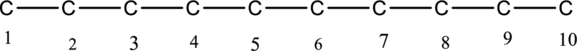
Then number and name the substituents in alphabetical order. The substituent methyl groups are bonded to second, third, fourth and fifth carbon position. Then prefix tetra is used to refer the four methyl groups. Then numbers has to be separated by using commas and the letters from numbers has to be separated by using dashes. Hence the structure of given IUPAC name 2,3,4,5-tetramethyldecane.
The substituent is attached to carbon chain is drawn below,
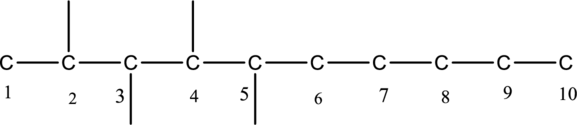
The structure of given compound 2,3,4,5, - tetramethyldecane is given below,
d.
Interpretation
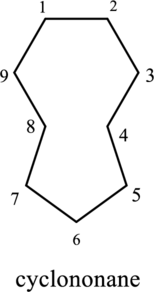
The structure of given IUPAC name cyclononane has to be drawn.
Concept Introduction:
Refer part a.
Explanation of Solution
The cyclic structure has nine carbon atoms and it is given as parent name. Hence the name can be given as cyclononane. In this compound there is no substituent group. There is no need to mention the numbering for single substituent.
The structure of given compound cyclononane is drawn below,
e.
Interpretation
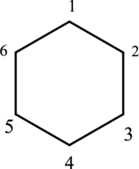
The structure of given IUPAC name 1,1,3-trimethylcyclohexane has to be drawn.
Concept Introduction:
Refer part a.
Explanation of Solution
The given IUPAC name 1,1,3-trimethylcyclohexane, where hexane denotes the six number of carbon atoms is present in the cyclic structure. Hence the parent name is given as hexane.
The structure for cyclohexane is a cyclic structure and is drawn below,
The three methyl substituents are bonded to first and third carbon. Then number and name the substituents in alphabetical order. The prefix tri- is used to represent three methyl groups. Hence the structure of given IUPAC name 3-ethylhexane.
The substituent attached to the cyclic structure is drawn below,
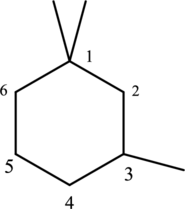
The structure of given compound 1,1,3 –trimethylcyclohexane is drawn below,
Want to see more full solutions like this?
Chapter 10 Solutions
Principles of General, Organic, Biological Chemistry
- Could you please explain whether my thinking is correct or incorrect regarding how I solved it? Please point out any mistakes in detail, with illustrations if needed.arrow_forwardWhat are the most proper reagents to achieve these products? سد 1. 2. OH ○ 1. BrMgC6H6; 2. H+ ○ 1. BrMgCH2CH2CH2CH2CH3; 2. H+ O 1. CH3CH2CHO; 2. H+ O 1. BrMgCH2CH3; 2. H+arrow_forwardProvide the IUPAC (systematic) name only for the following compound. Dashes, commas, and spaces must be correct. Harrow_forward
- Please use the nernst equation to genereate the Ion Selective Electrode Analysis standard curve within my excel spread sheet. Nernst Equation: E = Eo + m (ln a) Link: https://mnscu-my.sharepoint.com/:x:/g/personal/vi2163ss_go_minnstate_edu/EaREe1-PfGNKq1Cbink6kkYB5lBy05hEaE3mbGPUb22S6w?rtime=zQaSX3xY3Ugarrow_forwarda) b) c) H NaOH heat, dehydration + KOH heat, dehydration NaOH + (CH3)3CCHO heat, dehydration Pharrow_forwardshow mechanismarrow_forward
- Please draw by handarrow_forward3. Predict the major product and give a mechanism for the following reactions: (CH3)3COH/H₂SO4 a) b) NC CH₂O c) LOCH, (CH3)3COH/H2SO4 H,SO -OHarrow_forwardIndicate if the aldehyde shown reacts with the provided nucleophiles in acid or base conditions. a NaBH4 be Li eli -NH2 P(Ph3) f KCN g OH excess h CH3OH i NaCHCCH3arrow_forward
- Predict the major products of the following organic reaction: + A ? Some important notes: • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. Explanation Check Click and drag to start drawing a structure. C © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centearrow_forwardPolar solutes are most likely to dissolve into _____, and _____ are most likely to dissolve into nonpolar solvents. A. nonpolar solutes; polar solvents B. nonpolar solvents; polar solvents C. polar solvents; nonpolar solutes D. polar solutes; nonpolar solventsarrow_forwardDeducing the Peactants Can the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? ? Δ If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. If your answer is no, check the box under the drawing area instead. Explanation Check Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Xarrow_forward
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
