
Concept explainers
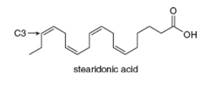
Linolenic acid(Table 10.2) and stearidonic acid are omega-3 fatty acids, unsaturated fatty acids that contain the first double bond located at C3, when numbering begins at the methyl end of the chain. Predict how the melting point of stearidonic acid compares with the melting points of linolenic acid and stearic acids. A current avenue of research is examining the use of soybean oil enriched in stearidonic acid as a healthier alternative to vegetable oils that contain fewer degrees of unsaturation.
Want to see the full answer?
Check out a sample textbook solution
Chapter 10 Solutions
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
Additional Science Textbook Solutions
Genetics: From Genes to Genomes
General, Organic, and Biological Chemistry - 4th edition
Fundamentals Of Thermodynamics
Loose Leaf For Integrated Principles Of Zoology
Human Anatomy & Physiology (2nd Edition)
Laboratory Manual For Human Anatomy & Physiology
- Don't used Ai solutionarrow_forwardBenzene-toluene equilibrium is often approximated as αBT = 2.34. Generate the y-x diagram for this relative volatility. Also, generate the equilibrium data using Raoult’s law, and compare your results to these.arrow_forwardGiven the most probable macrostate: s/k (K) Populations 300 4 200 8 100 16 0 32 Indicate how to demonstrate that the population of the levels is consistent with the Boltzmann distribution.arrow_forward
- Rank the following components in order of decreasing volatility: butane, n-pentane, iso-pentene (e.g., 3-methyl-1-butene), isoprene, pentanol? Briefly explain your answer.arrow_forwardViscosity of a liquid related to the activation energy.arrow_forwardVibrational contributions to internal energy and heat capacity1) are temperature independent2) are temperature dependentarrow_forward
- The approximation of calculating the partition function by integration instead of the summation of all the energy terms can only be done if the separation of the energy levels is much smaller than the product kT. Explain why.arrow_forwardExplain the meaning of: the electron partition function is equal to the degeneracy of the ground state.arrow_forward28. For each of the following species, add charges wherever required to give a complete, correct Lewis structure. All bonds and nonbonded valence electrons are shown. a. b. H H H H H :0-C-H H H H-C-H C. H H d. H-N-0: e. H H-O H-O H B=0 f. H—Ö—Ñ—Ö—H Norton Private Barrow_forward
- At 0oC and 1 atm, the viscosity of hydrogen (gas) is 8.55x10-5 P. Calculate the viscosity of a gas, if possible, consisting of deuterium. Assume that the molecular sizes are equal.arrow_forwardIndicate the correct option for the velocity distribution function of gas molecules:a) its velocity cannot be measured in any other way due to the small size of the gas moleculesb) it is only used to describe the velocity of particles if their density is very high.c) it describes the probability that a gas particle has a velocity in a given interval of velocitiesd) it describes other magnitudes, such as pressure, energy, etc., but not the velocity of the moleculesarrow_forwardIndicate the correct option for the velocity distribution function of gas molecules:a) its velocity cannot be measured in any other way due to the small size of the gas moleculesb) it is only used to describe the velocity of particles if their density is very high.c) it describes the probability that a gas particle has a velocity in a given interval of velocitiesd) it describes other magnitudes, such as pressure, energy, etc., but not the velocity of the moleculesarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax





