
Concept explainers
(a)
Interpretation: The constitutional isomer formed by the reaction of given alkene with [1]
Concept introduction: The compounds with same chemical formula but different arrangement of atoms in space are known as the constitutional isomer.
Hydration of
Answer to Problem 10.29P
The constitutional isomer formed by the reaction of given alkene with
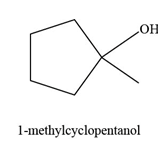
Figure 1
The constitutional isomer formed by the reaction of given alkene with
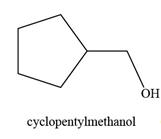
Figure 2
Explanation of Solution
The compounds with same chemical formula but different arrangement of atoms in space are known as the constitutional isomer.
Hydration of alkenes is one of the methods used for the formation of alcohol.
Hydroboration reaction is a two step reaction, which involves conversion of alkene into alcohol. This type of reaction follows Anti-Markovnikov’s rule.
In hydration reactions, the mode of addition is anti-addition, whereas in hydroboration-oxidation, the mode of addition is syn addition.
The given alkene is,
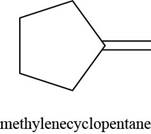
Figure 3
The product formed by the reaction of methylenecyclopentane with
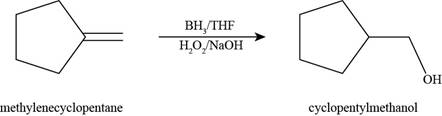
Figure 4
The general steps followed by the hydration reaction are stated below:
• First protonation of the alkene take place to generate the carbocation.
• Formation of protonated alcohol.
• Deprotonation.
The product formed by the reaction of methylenecyclopentane with
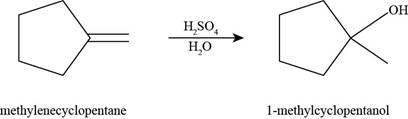
Figure 5
The constitutional isomer formed by the reaction of given alkenes with [1]
(b)
Interpretation: The constitutional isomer formed by the reaction of given alkenes with [1]
Concept introduction: The compounds with same chemical formula but different arrangement of atoms in space are known as the constitutional isomer.
Hydration of alkenes is one of the methods used for the formation of alcohol.
Answer to Problem 10.29P
The constitutional isomer formed by the reaction of given alkenes with
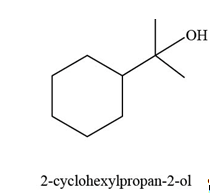
Figure 6
The constitutional isomer formed by the reaction of given alkenes with
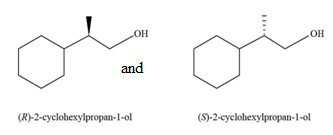
Figure 7
Explanation of Solution
The compounds with same chemical formula but different arrangement of atoms in space are known as the constitutional isomer.
Hydration of alkenes is one of the methods used for the formation of alcohol.
Hydroboration reaction is a two step reaction, which involves conversion of alkene into alcohol. This type of reaction follows Anti-Markovnikov’s rule.
In hydration reactions, the mode of addition is anti-addition, whereas in hydroboration-oxidation, the mode of addition is syn addition.
The given alkene is,
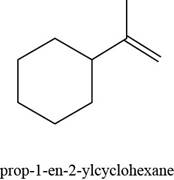
Figure 8
The product formed by the reaction of
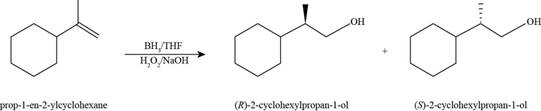
Figure 9
The general steps followed by the hydration reaction are stated below:
• First protonation of the alkene take place to generate the carbocation.
• Formation of protonated alcohol.
• Deprotonation.
The product formed by the reaction of
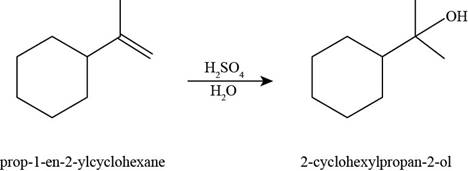
Figure 10
The constitutional isomer formed by the reaction of given alkenes with [1]
(c)
Interpretation: The constitutional isomer formed by the reaction of given alkene with [1]
Concept introduction: The compounds with same chemical formula but different arrangement of atoms in space are known as the constitutional isomer.
Hydration of alkenes is one of the methods used for the formation of alcohol.
Answer to Problem 10.29P
The constitutional isomer formed by the reaction of given alkene with
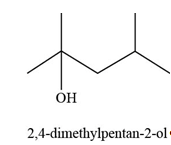
Figure 11
The constitutional isomer formed by the reaction of given alkene with
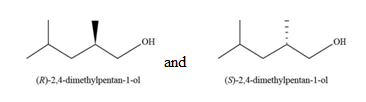
Figure 12
Explanation of Solution
The compounds with same chemical formula but different arrangement of atoms in space are known as the constitutional isomer.
Hydration of alkenes is one of the methods used for the formation of alcohol.
Hydroboration reaction is a two step reaction, which involves conversion of alkene into alcohol. This type of reaction follows Anti-Markovnikov’s rule.
In hydration reactions, the mode of addition is anti-addition, whereas in hydroboration-oxidation, the mode of addition is syn addition.
The given alkene is,
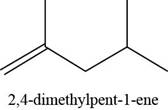
Figure 13
The product formed by the reaction of methylenecyclopentane with
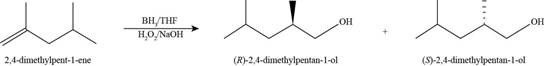
Figure 14
The general steps followed by the hydration reaction are stated below:
• First protonation of the alkene take place to generate the carbocation.
• Formation of protonated alcohol.
• Deprotonation.
The product formed by the reaction of methylenecyclopentane with
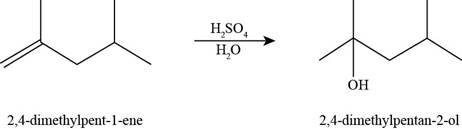
Figure 15
The constitutional isomer formed by the reaction of given alkenes with [1]
Want to see more full solutions like this?
Chapter 10 Solutions
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
- Imagine a four-dimensional world. In it, atoms would have one s orbital and four p orbitals in a given shell. (a) Describe the shape of the Periodic Table of the first 24 elements. (b) What elements would be the first two noble gases (use the names from our world that correspond to the atomic numbers).arrow_forwardThe electron affinity of thulium was measured by a technique called laser photodetachment electron spectroscopy. In this technique, a gaseous beam of anions of an element is bombarded with photons from a laser. The photons knock electrons off some of the anions, and the energies of the emitted electrons are detected. The incident radiation had a wavelength of 1064 nm, and the emitted electrons had an energy of 0.137 eV. Although the analysis is more complicated, we can obtain an estimate of the electron affinity from the energy difference between the photons and the emitted electrons. What is the electron affinity of thulium in electron volts and in kilojoules per mole?arrow_forwardBe sure to answer all parts. The following alkyne is treated with 03 followed by H₂O. Part 1: How many different compounds are formed in this process? 1 Part 2 out of 2 Draw the product of the reaction. draw structure ...arrow_forward
- Many fireworks use magnesium to burn, which releases a significant amount of energy. The heat released causes the oxide to glow, emitting white light. The color of this light can be changed by including nitrates and chlorides of elements that emit in the visible region of their spectra. One such compound is barium nitrate, which produces a yellow-green light. Excited barium ions generate light with wavelengths of 487 nm, 524 nm, 543 nm, and 578 nm. For each case, calculate: (a) the change in energy (in electron volts) of a barium atom and (b) the molar change in energy (in kilojoules per second).arrow_forwardClouds of hot, luminous interstellar hydrogen gas can be seen in some parts of the galaxy. In some hydrogen atoms, electrons are excited to quantum levels with n = 100 or higher. (a) Calculate the wavelength observed on Earth if the electrons fall from the level with n = 100 to one with n = 2. (b) In what series would this transition be found? (c) Some of these high-energy electrons fall into intermediate states, such as n = 90. Would the wavelengths of a transition from the state with n = 100 to one with n = 90 be longer or shorter than those in the Balmer series? Explain your answer.arrow_forwardIn the spectroscopic technique known as photoelectron spectroscopy (PES), ultraviolet radiation is directed at an atom or molecule. Electrons are ejected from the valence shell and their kinetic energies are measured. Since the energy of the incident ultraviolet photons is known and the kinetic energy of the ejected electron is measured, the ionization energy, I, can be deduced because total energy is conserved. (a) Show that the velocity, v, of the ejected electron and the frequency, n, of the incident radiation are related by hv = I + (1/2)mv^2? (b) Use this relation to calculate the ionization energy of a rubidium atom, knowing that light of wavelength 58.4 nm produces electrons with a velocity of 2,450 km/s Recall that 1 J = 1 kg.m^2/s^2arrow_forward
- I) In Millikan's experiment, each droplet observed by the technicians contained an even number of electrons. If they had been unaware of this limitation, how would it have affected their report of an electron's charge?II) Millikan measured the charge of an electron in electrostatic units, esu. The data he collected included the following series of charges found on oil drops: 9.60 X 10^-10 esu, 1.92 X 10^-9 esu; 2.40 X 10^-9 esu; 2.88 X 10^-9 esu; and 4.80 X 10^-9 esu. (a) From this series, find the probable charge of the electron in electrostatic units. (b) Estimate the number of electrons in an oil drop with a charge of 6.72 X 10^-9 esu. The actual charge (in Coulombs) of an electron is 1.602 X 10^-19 C. What is the relationship between esu and Coulombs?arrow_forwardmy ccc edu - Search X Quick Access X D2L Homepage - Spring 2025 x N Netflix X Dimensional Analysis - A x+ pp.aktiv.com Q ☆ X Question 59 of 70 The volume of 1 unit of plasma is 200.0 mL If the recommended dosage for adult patients is 10.0 mL per kg of body mass, how many units are needed for a patient with a body mass of 80.0 kg ? 80.0 kg 10.0 DAL 1 units X X 4.00 units 1 1 Jeg 200.0 DAL L 1 units X 200.0 mL = 4.00 units ADD FACTOR *( ) DELETE ANSWER RESET D 200.0 2.00 1.60 × 10³ 80.0 4.00 0.0400 0.250 10.0 8.00 & mL mL/kg kg units/mL L unit Q Search delete prt sc 111 110 19arrow_forwardIdentify the starting material in the following reaction. Click the "draw structure" button to launch the drawing utility. draw structure ... [1] 0 3 C10H18 [2] CH3SCH3 Harrow_forward
- In an equilibrium mixture of the formation of ammonia from nitrogen and hydrogen, it is found that PNH3 = 0.147 atm, PN2 = 1.41 atm and Pн2 = 6.00 atm. Evaluate Kp and Kc at 500 °C. 2 NH3 (g) N2 (g) + 3 H₂ (g) K₂ = (PN2)(PH2)³ = (1.41) (6.00)³ = 1.41 x 104arrow_forwardWhat alkene or alkyne yields the following products after oxidative cleavage with ozone? Click the "draw structure" button to launch the drawing utility. and two equivalents of CH2=O draw structure ...arrow_forwardH-Br Energy 1) Draw the step-by-step mechanism by which 3-methylbut-1-ene is converted into 2-bromo-2-methylbutane. 2) Sketch a reaction coordinate diagram that shows how the internal energy (Y- axis) of the reacting species change from reactants to intermediate(s) to product. Brarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





