
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
5th Edition
ISBN: 9781260170405
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10, Problem 10.72P
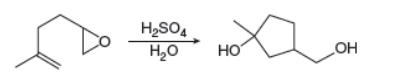
Draw a stepwise mechanism for the following reaction. This reaction combines two processes together: the
opening of an
double bond. (Hint: Begin the mechanism by protonating the epoxide ring.)
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Thermodynamic analysis of electrified interfaces.
Conduct a brief thermodynamic analysis of electrified interfaces. Gibbs model.
ELECTROCAPILAR EQUATION FOR IDEALLY POLARIZED ELECTRODES.
Chapter 10 Solutions
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
Ch. 10 - Prob. 10.1PCh. 10 - Problem 10.2 How many degrees of unsaturation are...Ch. 10 -
Problem 10.3 How many degrees of unsaturation...Ch. 10 - Give the IUPAC name for each alkene. abcdeCh. 10 - Give the IUPAC name for each polyfunctional...Ch. 10 - Problem 10.6 Label each C-C double bond as E or Z....Ch. 10 - Prob. 10.7PCh. 10 - Prob. 10.8PCh. 10 - Prob. 10.9PCh. 10 - Problem 10.10 Rank the following isomers in order...
Ch. 10 - Linolenic acidTable 10.2 and stearidonic acid are...Ch. 10 - Prob. 10.12PCh. 10 - Problem 10.13 What product is formed when each...Ch. 10 - Prob. 10.14PCh. 10 - Problem 10.15 Draw the products formed when each...Ch. 10 - Prob. 10.16PCh. 10 - Prob. 10.17PCh. 10 - Addition of HBr to which of the following alkenes...Ch. 10 - Problem 10.19 Draw the products, including...Ch. 10 - Prob. 10.20PCh. 10 - Problem 10.21 What two alkenes give rise to each...Ch. 10 - Prob. 10.22PCh. 10 - Problem 10.23 Draw the products of each reaction,...Ch. 10 - Problem 10.24 Draw all stereoisomers formed in...Ch. 10 - Prob. 10.25PCh. 10 - Problem 10.26 What alkylborane is formed from...Ch. 10 - Draw the products formed when each alkene is...Ch. 10 - What alkene can be used to prepare each alcohol as...Ch. 10 - Prob. 10.29PCh. 10 - Draw the products of each reaction using the two...Ch. 10 - Problem 10.31 Devise a synthesis of each compound...Ch. 10 - Give the IUPAC name for each compound. a.b.Ch. 10 - a Label the carbon-carbon double bond in A as E or...Ch. 10 - Prob. 10.34PCh. 10 - 10.35 Calculate the number of degrees of...Ch. 10 - Prob. 10.36PCh. 10 - Label the alkene in each drug as E or Z....Ch. 10 - Give the IUPAC name for each compound. a. c. e. b....Ch. 10 - Prob. 10.39PCh. 10 - 10.40 (a) Draw all possible stereoisomers of, and...Ch. 10 - Prob. 10.41PCh. 10 - 10.42 Now that you have learned how to name...Ch. 10 - Prob. 10.43PCh. 10 - Prob. 10.44PCh. 10 - Prob. 10.45PCh. 10 - Draw the products formed when (CH3)2C=CH2 is...Ch. 10 - What alkene can be used to prepare each alkyl...Ch. 10 - Prob. 10.48PCh. 10 - Draw the constitutional isomer formed in each...Ch. 10 - Prob. 10.50PCh. 10 - Draw all stereoisomers formed in each reaction. a....Ch. 10 - Draw the products of each reaction, including...Ch. 10 - Prob. 10.53PCh. 10 - Prob. 10.54PCh. 10 - Prob. 10.55PCh. 10 - 10.56 Draw a stepwise mechanism for the following...Ch. 10 - Prob. 10.57PCh. 10 - Draw a stepwise mechanism for the conversion of...Ch. 10 - Draw a stepwise mechanism that shows how all three...Ch. 10 - Less stable alkenes can be isomerized to more...Ch. 10 - Prob. 10.61PCh. 10 - Prob. 10.62PCh. 10 - Bromoetherification, the addition of the elements...Ch. 10 - Devise a synthesis of each product from the given...Ch. 10 - 10.65 Draw a synthesis of each compound from...Ch. 10 - 10.66 Explain why A is a stable compound but B is...Ch. 10 - Prob. 10.67PCh. 10 - Prob. 10.68PCh. 10 - 10.69 Lactones, cyclic esters such as compound A,...Ch. 10 - 10.70 Draw a stepwise mechanism for the following...Ch. 10 - 10.71 Like other electrophiles, carbocations add...Ch. 10 - 10.72 Draw a stepwise mechanism for the...
Additional Science Textbook Solutions
Find more solutions based on key concepts
How does the removal of hydrogen atoms from nutrient molecules result in a loss of energy from the nutrient mol...
SEELEY'S ANATOMY+PHYSIOLOGY
60. The solar system is 25,000 light years from the center of our Milky Way galaxy. One light year is the dista...
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Why is it necessary to be in a pressurized cabin when flying at 30,000 feet?
Anatomy & Physiology (6th Edition)
On what molecule does the anticodon appear? Explain the role of this molecule in protein synthesis.
Human Physiology: An Integrated Approach (8th Edition)
Why is it unlikely that two neighboring water molecules would be arranged like this?
Campbell Biology (11th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Briefly state the electrocapillary equation for ideally polarized electrodes.arrow_forwardWhat is surface excess according to the Gibbs model?arrow_forwardUsing Benzene as starting materid show how each of the Following molecules Contel Ve syntheswed CHI 9. b -50311 с CHY 503H Ночто d. อ •NOV e 11-0-650 NO2arrow_forward
- The molecule PYRIDINE, 6th electrons and is therefore aromatre and is Assigned the Following structure contering Since aromatk moleculoy undergo electrophilic anomatic substitution, Pyridine shodd undergo The Following reaction + HNO3 12504 a. write all of the possible Mononitration Products that could Result From this reaction 18. Bared upon the reaction mechanison determime which of these producty would be the major Product of the hegetionarrow_forwarda. Explain Why electron withdrawing groups tend to be meta-Directors. Your answer Should lyclude all apropriate. Resonance contributing Structures fo. Explain why -ll is an outho -tura drccton even though chlorine has a very High Electronegativityarrow_forward9. Write Me product as well as the reaction Mechanism For each of the Following Vanctions +H₂504 4.50+ T C. +212 Fellz 237 b. Praw the potential energy Diagrams For each OF Mese Rauctions and account For any differences that appear in the two potential Puergy Diagrams which of here two reactions 19 Found to be Reversable, Rationalice your answer based upon the venation mechanisms and the potential energy diagrams.arrow_forward
- 9. Write Me product as well as the reaction Mechanism For each of the Following Veritious +H2504 4.50+ + 1/₂ Felly ◎+ 7 b. Praw he potential energy Diagrams For each OF Mese Ronctions and account for any differences that appeak in the two potential Puergy Diagramsarrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Incorrect, 3 attempts remaining 1. excess Br2, NaOH 2. neutralizing workup Qarrow_forwardGiven the electrode Pt | Ag | Ag+ (aq), describe it.arrow_forward
- At 25°C, the reaction Zn2+ + 2e ⇄ Zn has a normal equilibrium potential versus the saturated calomel electrode of -1.0048 V. Determine the normal equilibrium potential of Zn versus the hydrogen electrode.Data: The calomel electrode potential is E° = 0.2420 V versus the normal hydrogen electrode.arrow_forwardElectrochemistry. State the difference between E and E0.arrow_forwardIn an electrolytic cell, the positive pole is always assumed to be on the right side of the battery notation. Is that correct?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Characteristic Reactions of Benzene and Phenols; Author: Linda Hanson;https://www.youtube.com/watch?v=tjEqEjDd87E;License: Standard YouTube License, CC-BY
An Overview of Aldehydes and Ketones: Crash Course Organic Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=-fBPX-4kFlw;License: Standard Youtube License