
Concept explainers
(a)
Interpretation: The structure corresponding to the given
Concept introduction: The systematic naming of organic compound is given by
Rules for writing structural formula from IUPAC are:
1. First identify the word root for the given compound.
2. The suffix used in the compound like –ene.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
The use of prefix
Answer to Problem 10.39P
The structure corresponding to
Explanation of Solution
The given IUPAC name is
Rules for writing structural formula from IUPAC are
1. First identify the word root for the given compound.
2. The suffix used in the compound like –ene.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
The use of prefix
The given name is
Thus, the correct structure of
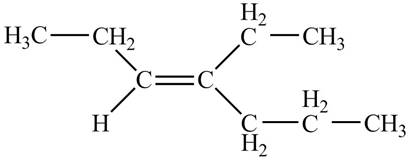
Figure 1
The structure corresponding to
(b)
Interpretation: The structure corresponding to the given
Concept introduction: The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing structural formula from IUPAC are:
1. First identify the word root for the given compound.
2. The suffix used in the compound like –ene.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
Answer to Problem 10.39P
The structure corresponding to
Explanation of Solution
Rules for writing structural formula from IUPAC are
1. First identify the word root for the given compound.
2. The suffix used in the compound like –ene.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
The given name is
Thus, the correct structure of
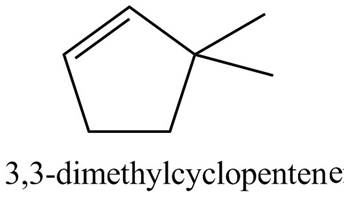
Figure 2
The structure corresponding to
(c)
Interpretation: The structure corresponding to
Concept introduction: The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing structural formula from IUPAC are:
1. First identify the word root for the given compound.
2. The suffix used in the compound like –ene.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
Answer to Problem 10.39P
The structure corresponding to
Explanation of Solution
Rules for writing structural formula from IUPAC are
1. First identify the word root for the given compound.
2. The suffix used in the compound like –ene.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
The given name is
Thus, the correct structure of
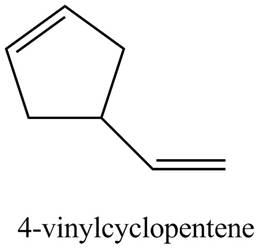
Figure 3
The structure corresponding to
(d)
Interpretation: The structure corresponding to
Concept introduction: The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing structural formula from IUPAC are:
1. First identify the word root for the given compound.
2. The suffix used in the compound like –ene.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
The use of prefix
Answer to Problem 10.39P
The structure corresponding to
Explanation of Solution
Rules for writing structural formula from IUPAC are
1. First identify the word root for the given compound.
2. The suffix used in the compound like –ene.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
The use of prefix
The given name is
Thus, the correct structure of
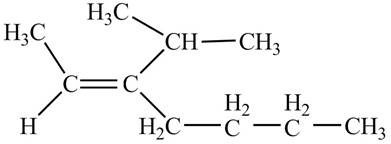
Figure 4
The structure corresponding to
(e)
Interpretation: The structure corresponding to
Concept introduction: The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing structural formula from IUPAC are:
1. First identify the word root for the given compound.
2. The suffix used in the compound like –ene.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
Answer to Problem 10.39P
The structure corresponding to
Explanation of Solution
Rules for writing structural formula from IUPAC are
1. First identify the word root for the given compound.
2. The suffix used in the compound like –ene.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
The given name is
Thus, the two correct structure of
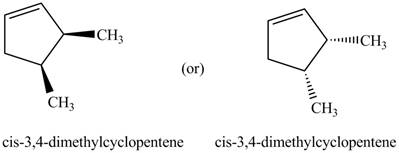
Figure 5
The structure corresponding to
(f)
Interpretation: The structure corresponding to
Concept introduction: The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing structural formula from IUPAC are:
1. First identify the word root for the given compound.
2. The suffix used in the compound like –ene.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
Answer to Problem 10.39P
The structure corresponding to
Explanation of Solution
Rules for writing structural formula from IUPAC are
1. First identify the word root for the given compound.
2. The suffix used in the compound like –ene.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
The given name is
Thus, the correct structure of
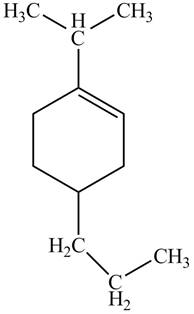
Figure 6
The structure corresponding to
(g)
Interpretation: The structure corresponding to
Concept introduction: The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing structural formula from IUPAC are:
1. First identify the word root for the given compound.
2. The suffix used in the compound like –ene.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
Answer to Problem 10.39P
The structure corresponding to
Explanation of Solution
Rules for writing structural formula from IUPAC are
1. First identify the word root for the given compound.
2. The suffix used in the compound like –ene.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
The given name is
Thus, the correct structure of
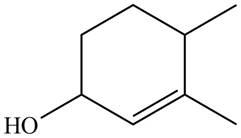
Figure 7
The structure corresponding to
(h)
Interpretation: The structure corresponding to
Concept introduction: The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing structural formula from IUPAC are:
1. First identify the word root for the given compound.
2. The suffix used in the compound like –ene.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
Answer to Problem 10.39P
The structure corresponding to
Explanation of Solution
Rules for writing structural formula from IUPAC are
1. First identify the word root for the given compound.
2. The suffix used in the compound like –ene.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
The given name is
Thus, the correct structure of
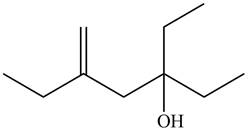
Figure 8
The structure corresponding to
Want to see more full solutions like this?
Chapter 10 Solutions
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
- What is the IUPAC name of the following compound? CH₂CH₂ H CI H₂CH₂C H CH₂ Selected Answer: O (35,4R)-4 chloro-3-ethylpentane Correctarrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electrons-pushing arrows for the following reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. I I I H Select to Add Arrows HCI, CH3CH2OHarrow_forward
- Curved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and the follow the arrows to draw the intermediate and product in this reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the curved arrows to draw the intermediates and product of the following reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the arrows to draw the intermediate and the product in this reaction or mechanistic step(s).arrow_forward
- Look at the following pairs of structures carefully to identify them as representing a) completely different compounds, b) compounds that are structural isomers of each other, c) compounds that are geometric isomers of each other, d) conformers of the same compound (part of structure rotated around a single bond) or e) the same structure.arrow_forwardGiven 10.0 g of NaOH, what volume of a 0.100 M solution of H2SO4 would be required to exactly react all the NaOH?arrow_forward3.50 g of Li are combined with 3.50 g of N2. What is the maximum mass of Li3N that can be produced? 6 Li + N2 ---> 2 Li3Narrow_forward
- 3.50 g of Li are combined with 3.50 g of N2. What is the maximum mass of Li3N that can be produced? 6 Li + N2 ---> 2 Li3Narrow_forwardConcentration Trial1 Concentration of iodide solution (mA) 255.8 Concentration of thiosulfate solution (mM) 47.0 Concentration of hydrogen peroxide solution (mM) 110.1 Temperature of iodide solution ('C) 25.0 Volume of iodide solution (1) used (mL) 10.0 Volume of thiosulfate solution (5:03) used (mL) Volume of DI water used (mL) Volume of hydrogen peroxide solution (H₂O₂) used (mL) 1.0 2.5 7.5 Time (s) 16.9 Dark blue Observations Initial concentration of iodide in reaction (mA) Initial concentration of thiosulfate in reaction (mA) Initial concentration of hydrogen peroxide in reaction (mA) Initial Rate (mA's)arrow_forwardDraw the condensed or line-angle structure for an alkene with the formula C5H10. Note: Avoid selecting cis-/trans- isomers in this exercise. Draw two additional condensed or line-angle structures for alkenes with the formula C5H10. Record the name of the isomers in Data Table 1. Repeat steps for 2 cyclic isomers of C5H10arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





