
Pushing Electrons
4th Edition
ISBN: 9781133951889
Author: Weeks, Daniel P.
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 9EQ
Methanethiol
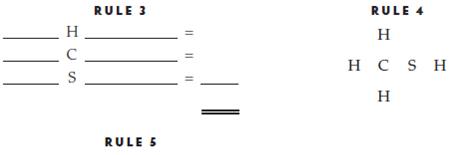
No. of electrons in structure _____
No. of valence electrons _____
Structure is ___________
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
4. True or false: This skeletal structure represents a saturated fatty acid. Ini to
0
fale) me
OH
faistong st
By malonic or acetylacetic synthesis, synthesize 5-Methyl-2-hexanone (with the formulas of the compounds).
QUESTION: Answer Question 5: 'Calculating standard error of regression' by filling in all the empty green boxes
*The values are all provided in the first photo attached*
Chapter 1 Solutions
Pushing Electrons
Ch. 1 - 1. Hydrogen is a Group I element and each...Ch. 1 - Methanol has the molecular formula CH4O. Its...Ch. 1 - 3. The skeleton of chloromethane is...Ch. 1 - 4. Methanol’s skeleton is
Connecting all bonded...Ch. 1 - 5. The structure for chloromethane is
It...Ch. 1 - Prob. 6EQCh. 1 - 7. Dimethyl ether
No. of electrons in...Ch. 1 - Methylamine (CH5N) No. of electrons in structure...Ch. 1 - Methanethiol (CH4S) No. of electrons in structure...Ch. 1 - Methylal (C3H8O2) No. of electrons in structure...
Ch. 1 - Prob. 11EQCh. 1 - Adding electrons to the skeleton by making single...Ch. 1 - This is done by removing an unshared pair from...Ch. 1 - Prob. 14EQCh. 1 - Prob. 15EQCh. 1 - Prob. 16EQCh. 1 - The skeleton of acetyl chloride is . Write the...Ch. 1 - Three constitutional isomers exist for the formula...Ch. 1 - A number of constitutional isomers exist for the...Ch. 1 - Using the method outlined above, derive the...Ch. 1 - Prob. 21EQCh. 1 - Prob. 22EQCh. 1 - Prob. 23EQCh. 1 - Prob. 24EQCh. 1 - The skeleton of benzyldimethylamine is
The...Ch. 1 - The skeleton is benzaldoxime is The number of...Ch. 1 - Prob. 27EQCh. 1 - Derive Lewis structures for the compounds below....Ch. 1 - Prob. 29EQCh. 1 - Derive Lewis structures for the compounds below....Ch. 1 - Prob. 31EQCh. 1 - Derive Lewis structures for the compounds below....Ch. 1 - The Lewis structure of acetone is Circling the...Ch. 1 - Chloromethane has the Lewis...Ch. 1 - In the Lewis structure for chloromethane, the...Ch. 1 - Prob. 36EQCh. 1 - The oxygen atom in acetone possesses ____ unshared...Ch. 1 - Nitrobenzene has the skeleton
The number of...Ch. 1 - Prob. 39EQCh. 1 - Compute and add on the formal charges I these...Ch. 1 - Prob. 41EQCh. 1 - Prob. 42EQCh. 1 - Prob. 43EQCh. 1 - Prob. 44EQCh. 1 - Prob. 45EQCh. 1 - Prob. 46EQCh. 1 - Prob. 47EQCh. 1 - Compute and add on the formal charges in these...Ch. 1 - Prob. 49EQCh. 1 - Prob. 50EQCh. 1 - The n-propyl cation can be formed from a molecule...Ch. 1 - Prob. 52EQCh. 1 - Prob. 53EQCh. 1 - Methanol, CH3OH, is a compound in which the formal...Ch. 1 - When a proton becomes bonded to diethyl ether, by...Ch. 1 - Tetrahydrofuran has the structure
When a proton...Ch. 1 - Prob. 57EQCh. 1 - Prob. 58EQCh. 1 - The structure of pyridine is
When a proton...Ch. 1 - The carbon atom owns one electron from each of ...Ch. 1 - The n-butyl anion can be formed from When the CLi...Ch. 1 - The isobutyl anion can be formed from When the CNa...Ch. 1 - Prob. 63EQCh. 1 - Ethanol, , is a compound in which the formal...Ch. 1 - The loss of a proton attached to the oxygen atom...Ch. 1 - A very strong base can remove a proton from...Ch. 1 - Prob. 67EQCh. 1 - Prob. 68EQCh. 1 - Prob. 69EQCh. 1 - The homolysis of the OO bond in diacetyl peroxide...Ch. 1 - Prob. 71EQCh. 1 - Prob. 72EQCh. 1 - Prob. 73EQCh. 1 - Prob. 74EQCh. 1 - Prob. 75EQCh. 1 - Heterolytic cleavage of the CO bond to yield a...Ch. 1 - Prob. 77EQCh. 1 - Prob. 78EQCh. 1 - Prob. 79EQCh. 1 - Prob. 80EQ
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the formula for 3-chlorobenzoic acetic anhydride.arrow_forwardBy malonic or acetylacetic synthesis, synthesize 2-methylbutanoic acid (indicate the formulas of the compounds).arrow_forwardObtain 2-methylbutanoic acid by malonic or acetylacetic synthesis (indicate the formulas of the compounds involved).arrow_forward
- EFFICIENTS SAMPLE READINGS CONCENTRATIONS Pigiadient) TOMATO SAUCE (REGULAR) TOMATO (REDUCED SALT) TOMATO SAUCE (REGULAR) TOMATO (REDUCED SALT) 58 6.274 3.898 301.7 151.2 14150 5.277 3.865 348.9 254.8 B 5.136 3.639 193.7 85.9 605 4.655 3.041 308.6 199.6 05 5.135 3.664 339.5 241.4 0139 4.676 3.662 160.6 87.6 90148 5.086 3.677 337.7 242.5 0092 6.348 3.775 464.7 186.4 PART3 5.081 3.908 223.5 155.8 5.558 3.861 370.5 257.1 4.922 3.66 326.6 242.9 4.752 3.641 327.5 253.3 50 5.018 3.815 336.1 256.0 84 4.959 3.605 317.9 216.6 38 4.96 3.652 203.8 108.7 $3 5.052 3.664 329.8 239.0 17 5.043 3.767 221.9 149.7 052 5.058 3.614 331.7 236.4 5.051 4.005 211.7 152.1 62 5.047 3.637 309.6 222.7 5.298 3.977 223.4 148.7 5.38 4.24 353.7 278.2 5 5.033 4.044 334.6 268.7 995 4.706 3.621 305.6 234.4 04 4.816 3.728 340.0 262.7 16 4.828 4.496 304.3 283.2 0.011 4.993 3.865 244.7 143.6 AVERAGE STDEV COUNT 95% CI Confidence Interval (mmol/L) [Na+] (mg/100 mL) 95% Na+ Confidence Interval (mg/100 mL)arrow_forwardIf we have two compounds: acetone (CH₃COCH₃) and acetic acid (CH₃COOH), applying heat to them produces an aldol condensation of the two compounds. If this is correct, draw the formula for the final product.arrow_forwardIf we have two compounds: acetone (CH3COCH3) and acetic acid (CH3COOH); if we apply heat (A), what product(s) are obtained?arrow_forward
- QUESTION: Fill out the answers to the empty green boxes attached in the image. *Ensure you all incorporate all 27 values (per column)*arrow_forwardYou need to make a buffer by dissolving benzoic acid and sodium benzoate in water. What is the mass of benzoic acid that you would weigh out, in mg, to create 50 mL of a buffer at pH = 4.7 that will change pH no more than 0.10 units with the addition of 0.001 moles of acid or base? Enter just the answer without the units (mg) - just the number will do!arrow_forwardDraw the formula for 3-isopropylcyclopentane-1-carbonyl chloride.arrow_forward
- QUESTION: Fill out the answers to the empty green boxes attached in the image. *Ensure you all incorporate all 27 values (per column)*arrow_forwardComplete the following reactions- hand written pleasearrow_forwardGive the organic product: O A O B Ос ○ D -NH–CH3 + CH3 CH3 NEN C ? A CH3 CH3 NH- CH3 B CH3 CH3 N=N- C CH3 CH3 N=NNH CH3 D CH3 N=N CH3 NHCH3 LNH CHOarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning
Types of bonds; Author: Edspira;https://www.youtube.com/watch?v=Jj0V01Arebk;License: Standard YouTube License, CC-BY