
Concept explainers
The skeleton is benzaldoxime is
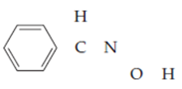
The number of valence electrons is as follows: from the phenyl group. ___; from each of two hydrogens, ___; from the carbon atom, ___; from the nitrogen atom, ___; and from the oxygen atom, ___, for a total of ___. Filling in the skeleton with single bonds and adding the appropriate unshared pairs gives
_____________________
The number of electrons in the
_____________________
in which carbon, nitrogen, and oxygen have their customary valences of ___, ___, and respectively. The alternative structure with a double bond between nitrogen and oxygen is
_____________________
This structure is not acceptable because it requires carbon and oxygen to exhibit the unfamiliar valences of ___ and ___.
Derive Lewis structures for the compounds below.
Want to see the full answer?
Check out a sample textbook solution
Chapter 1 Solutions
Pushing Electrons
- What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.arrow_forwardWhat is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forward
- The temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forward

 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER


