
Pushing Electrons
4th Edition
ISBN: 9781133951889
Author: Weeks, Daniel P.
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 13EQ
This is done by removing an unshared pair from each of two adjacent atoms and adding one electron pair as a second bond between the atoms. Each such operation reduces the number of electrons in the trial structure by two. Removing the unshared pairs of electrons on the carbon atoms and adding a second carbon–carbon bond gives
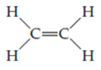
a structure in which there are four electrons involved in a double bond between the carbon atoms. The trial structure now contains electrons ____ and is correct.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Results
Search Results
Best Free Coursehero Unloc xb Success Confirmation of Q x
O Google Pas
alekscgi/x/lsl.exe/1o_u-IgNslkr 7j8P3jH-IQs_pBanHhvlTCeeBZbufu BYTI0Hz7m7D3ZcHYUt80XL-5alyVpwDXM TEZayFYCavJ17dZtpxbFD0Qggd1J
O States of Matter
Using a phase diagram to find a phase transition temperature or pressure
Gabr
3/5
he pressure above a pure sample of solid Substance X at 101. °C is lowered. At what pressure will the sample sublime? Use the phase diagram of X below to
nd your answer.
pressure (atm)
24-
12
solid
liquid
gas
200
400
temperature (K)
600
ote: your answer must be within 0.15 atm of the exact answer to be graded correct.
atm
Thanation
Check
© 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center I
Q Search
L³
ملة
301.7
348.9
193.7
308.6
339.5
160.6
337.7
464.7
223.5
370.5
326.6
327.5
336.1
317.9
203.8
329.8
221.9
331.7
211.7
309.6
223.4
353.7
334.6
305.6
340.0
304.3
244.7
QUESTION: Using this group of data on regular tomato sauce concentration readings answer the following questions:
1. 95% Cl Confidence Interval (mmol/L)
2. [Na+] (mg/100 mL)
3. 95% Na+ Confidence Interval (mg/100 mL)
Search Results
Search Results
Best Free Coursehero Unlo x b Success Confirmation of Q
aleks.com/alekscgi/x/sl.exe/10_u-lgNslkr7j8P3jH-IQs_pBan HhvlTCeeBZbufu BYTIOHz7m7D3ZcHYUt80XL-5alyVpwDXM TEZayFYCav
States of Matter
Using a phase diagram to find a phase transition temperature or pressure
Use the phase diagram of Substance X below to find the temperature at which X turns to a gas, if the pressure above the solid is 3.7 atm.
pressure (atm)
0.
32-
16
solid
liquid
gas
200
temperature (K)
Note: your answer must be within 20 °C of the exact answer to be graded correct.
Дос
X
Chapter 1 Solutions
Pushing Electrons
Ch. 1 - 1. Hydrogen is a Group I element and each...Ch. 1 - Methanol has the molecular formula CH4O. Its...Ch. 1 - 3. The skeleton of chloromethane is...Ch. 1 - 4. Methanol’s skeleton is
Connecting all bonded...Ch. 1 - 5. The structure for chloromethane is
It...Ch. 1 - Prob. 6EQCh. 1 - 7. Dimethyl ether
No. of electrons in...Ch. 1 - Methylamine (CH5N) No. of electrons in structure...Ch. 1 - Methanethiol (CH4S) No. of electrons in structure...Ch. 1 - Methylal (C3H8O2) No. of electrons in structure...
Ch. 1 - Prob. 11EQCh. 1 - Adding electrons to the skeleton by making single...Ch. 1 - This is done by removing an unshared pair from...Ch. 1 - Prob. 14EQCh. 1 - Prob. 15EQCh. 1 - Prob. 16EQCh. 1 - The skeleton of acetyl chloride is . Write the...Ch. 1 - Three constitutional isomers exist for the formula...Ch. 1 - A number of constitutional isomers exist for the...Ch. 1 - Using the method outlined above, derive the...Ch. 1 - Prob. 21EQCh. 1 - Prob. 22EQCh. 1 - Prob. 23EQCh. 1 - Prob. 24EQCh. 1 - The skeleton of benzyldimethylamine is
The...Ch. 1 - The skeleton is benzaldoxime is The number of...Ch. 1 - Prob. 27EQCh. 1 - Derive Lewis structures for the compounds below....Ch. 1 - Prob. 29EQCh. 1 - Derive Lewis structures for the compounds below....Ch. 1 - Prob. 31EQCh. 1 - Derive Lewis structures for the compounds below....Ch. 1 - The Lewis structure of acetone is Circling the...Ch. 1 - Chloromethane has the Lewis...Ch. 1 - In the Lewis structure for chloromethane, the...Ch. 1 - Prob. 36EQCh. 1 - The oxygen atom in acetone possesses ____ unshared...Ch. 1 - Nitrobenzene has the skeleton
The number of...Ch. 1 - Prob. 39EQCh. 1 - Compute and add on the formal charges I these...Ch. 1 - Prob. 41EQCh. 1 - Prob. 42EQCh. 1 - Prob. 43EQCh. 1 - Prob. 44EQCh. 1 - Prob. 45EQCh. 1 - Prob. 46EQCh. 1 - Prob. 47EQCh. 1 - Compute and add on the formal charges in these...Ch. 1 - Prob. 49EQCh. 1 - Prob. 50EQCh. 1 - The n-propyl cation can be formed from a molecule...Ch. 1 - Prob. 52EQCh. 1 - Prob. 53EQCh. 1 - Methanol, CH3OH, is a compound in which the formal...Ch. 1 - When a proton becomes bonded to diethyl ether, by...Ch. 1 - Tetrahydrofuran has the structure
When a proton...Ch. 1 - Prob. 57EQCh. 1 - Prob. 58EQCh. 1 - The structure of pyridine is
When a proton...Ch. 1 - The carbon atom owns one electron from each of ...Ch. 1 - The n-butyl anion can be formed from When the CLi...Ch. 1 - The isobutyl anion can be formed from When the CNa...Ch. 1 - Prob. 63EQCh. 1 - Ethanol, , is a compound in which the formal...Ch. 1 - The loss of a proton attached to the oxygen atom...Ch. 1 - A very strong base can remove a proton from...Ch. 1 - Prob. 67EQCh. 1 - Prob. 68EQCh. 1 - Prob. 69EQCh. 1 - The homolysis of the OO bond in diacetyl peroxide...Ch. 1 - Prob. 71EQCh. 1 - Prob. 72EQCh. 1 - Prob. 73EQCh. 1 - Prob. 74EQCh. 1 - Prob. 75EQCh. 1 - Heterolytic cleavage of the CO bond to yield a...Ch. 1 - Prob. 77EQCh. 1 - Prob. 78EQCh. 1 - Prob. 79EQCh. 1 - Prob. 80EQ
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the reaction below to answer the following questions: Acetoacetic ester can be prepared by the Claisen self-condensation reaction of ethyl acetate. 1. NaOEt, EtOH H&C OCH CH3 2 H30 H3C CH2 OCH2CH3 A. Write the complete stepwise mechanism for this reaction. Show all electron flow with arrows and draw all intermediate structures. B. Ethyl acetate can be prepared from ethanol as the only organic starting material. Show all reagents and structures for all intermediates in this preparation. C. Give the structures of the ester precursors for the following Claisen condensation product and formulate the reaction. OEtarrow_forwardUse the phase diagram of Substance X below to find the temperature at which X turns to a gas, if the pressure above the solid is 3.7 atm. pressure (atm) 32 16 solid liquid gas 0 0 200 temperature (K) Note: your answer must be within 20 °C of the exact answer to be graded correct. Шос ☑ كarrow_forwardStarting from bromoethane, how could you prepare the following compounds: a. Ethanol. b. Acetaldehyde f. Acetone. e. 2-Propanol i. Acetoacetic ester. d. 2-Bromoacetic acid. c. Acetic acid g. Acetamide. j. Ethylmalonate k. Gama ketoacid. h. Ethyl magnesium bromide.arrow_forward
- - The pressure above a pure sample of solid Substance X at 60. °C is raised. At what pressure will the sample melt? Use the phase diagram of X below to find your answer. pressure (atm) 02 0.4 solid Hliquid gas 0 0 200 400 600 temperature (K) Note: your answer must be within 0.025 atm of the exact answer to be graded correct. ☐ atmarrow_forward15. What is the order of decreasing reactivity towards nucleophilic acyl substitution for the carboxylic acid derivatives? (most reactive first) 0 O H3C COC CH3 H₂C C N(CH3)2 H3C C OCH3 A. a. I, 11, 111, b. I, III, IV, II C. II, IV, III, I ° (CH3)2CH C OCH3 IV d. II, I, III, IV B. R COCR 0 0 0 13= RC NH2 RC OR RC CI === IV a. I, III, II, IV b. II, III, I, IV C. III, II, I, IV d. IV, I, III, IIarrow_forwardDraw the formula of the product obtained by reacting D-Tallose with bromine water.arrow_forward
- Choose the best reagent(s) for carrying out the following conversions from the list below. Place the letter corresponding to the best choice in the blank to the left of the conversion. a. KMnO4, H3O+ b. Tollens' Reagent [oxidizing reagent] C. NaBH4, ethanol d. 1. BH3 2. H3O+ e. 1. CH3MgBr, ether 2. H3O+ f. CrO3, H2SO4, H₂O g. 1. Mg, ether 2. CO2 3. H3O+ h. 1. NaCN 2. H2SO4, H2, heat i. O3, then Zn and HOAC j. CH₂I A. B. C. CH CH=CHCH2COOH Br CEN CH COOH + HOOCCH COOH COOH 010 CH3arrow_forwardDraw the structures for each of the intermediates in the boxes provided for the synthesis below. OCH3 Fe HO HNO (CHOO pynding H₂504 LHNO2 NACH-I Fa H₂O HCL HNO 180arrow_forwardProvide structure(s) for the starting material(s), reagent(s) or the major organic product(s) of each of the following reactions or sequences of reactions. Show all relevant stereochemistry [three only] A. o 11 (CH3)CH — C— C ether (CH3)2CH-C-O-C-CH3 B. CH3 CHy CI Staf OH C. HC OCHS + H₂Oarrow_forward
- Consider the reaction sequence below to answer the following questions: EtO Compound X 1. NaOEt, EtOH OEt Br CO₂Et NaOEt, EtOH Compound Z CO₂Et Compound Y A. Compound X, diethyl propanedioate, is more commonly known as a. ethyl acetoacetate b. acetoacetic ester C. oxalic ester d. malonic ester 3. Write the complete stepwise mechanism for the conversion of Compound X into Compound Y. Show all electron flow with arrows and draw all intermediate structures.arrow_forwardClassify each of the following nitrogen atoms in the following compounds as primary, secondary, tertiary, or quaternary [three only] CH3 HO-CHCHNHCH3 A. B. C. H&C CH3 D. HO phedrine CH2CHCH3 amphetamine NH₂ mepiquat chloride faxofenadine OH H&C CH CO₂Harrow_forwardDraw the structure of the aldol self-condensation product for each of the following compounds. If a compound does not undergo aldol self-condensation, explain why it does not. A. B. CHICHCH₂OH CH3CHCH2CH CH3 CH3 C. CH 30 H3C-C-C-H CH3 questionsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License