
ORGANIC CHEMISTRY-STUD.SOLNS.MAN+SG(LL)
4th Edition
ISBN: 9781119659587
Author: Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 1, Problem 55PP
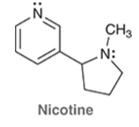
Nicotine is an addictive substance found in tobacco. Identify the hybridization state and geometry of each the nitrogen atoms in nicotine:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Could you please turn this into a complete Lewis dot structure formula for me so I can visualize it more clearly? and then do the explaining for the resonance structures that were given please.
Could you please turn this into a complete Lewis dot structure formula for me so I can visualize it more clearly? and then do the explaining for the question.
please solve. If the answer is "no error" and it asks me to type something, and i typed a-helix, its always wrong.
Chapter 1 Solutions
ORGANIC CHEMISTRY-STUD.SOLNS.MAN+SG(LL)
Ch. 1.2 - Prob. 1LTSCh. 1.2 - Prob. 2ATSCh. 1.2 - Prob. 2LTSCh. 1.3 - Prob. 3LTSCh. 1.3 - Prob. 4PTSCh. 1.3 - Prob. 5PTSCh. 1.4 - Prob. 4LTSCh. 1.4 - Prob. 7PTSCh. 1.4 - Prob. 8PTSCh. 1.4 - Prob. 9ATS
Ch. 1.5 - Prob. 5LTSCh. 1.5 - Prob. 10PTSCh. 1.5 - Prob. 11ATSCh. 1.5 - Prob. 12ATSCh. 1.6 - Prob. 6LTSCh. 1.6 - Prob. 14ATSCh. 1.7 - Prob. 7LTSCh. 1.7 - Prob. 17ATSCh. 1.10 - Prob. 18CCCh. 1.10 - Prob. 20CCCh. 1.10 - Prob. 8LTSCh. 1.10 - Prob. 21PTSCh. 1.10 - Nemotin is a compound that was first isolated from...Ch. 1.10 - Prob. 23CCCh. 1.11 - Prob. 9LTSCh. 1.11 - Prob. 24PTSCh. 1.11 - Prob. 25PTSCh. 1.11 - Prob. 26PTSCh. 1.11 - Prob. 27ATSCh. 1.12 - Prob. 10LTSCh. 1.12 - Prob. 29ATSCh. 1.13 - Prob. 11LTSCh. 1.13 - Prob. 31ATSCh. 1 - Prob. 32PPCh. 1 - Prob. 33PPCh. 1 - Prob. 34PPCh. 1 - Prob. 35PPCh. 1 - Prob. 36PPCh. 1 - Prob. 37PPCh. 1 - Prob. 38PPCh. 1 - Prob. 39PPCh. 1 - Prob. 40PPCh. 1 - Prob. 41PPCh. 1 - Prob. 42PPCh. 1 - Prob. 44PPCh. 1 - Prob. 45PPCh. 1 - Prob. 46PPCh. 1 - Prob. 47PPCh. 1 - Prob. 48PPCh. 1 - Prob. 49PPCh. 1 - Prob. 50PPCh. 1 - Prob. 51PPCh. 1 - Prob. 52PPCh. 1 - Prob. 53PPCh. 1 - Prob. 54PPCh. 1 - Nicotine is an addictive substance found in...Ch. 1 - Prob. 56PPCh. 1 - Prob. 57PPCh. 1 - Prob. 59PPCh. 1 - Prob. 63ASPCh. 1 - Prob. 64ASPCh. 1 - Prob. 66ASPCh. 1 - Prob. 69ASPCh. 1 - Prob. 71ASPCh. 1 - Prob. 72ASPCh. 1 - Prob. 75IP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Can you please solve and explain this for me in a simple way? I cant seem to comprehend this problem.arrow_forwardPart I. Problem solving. Include all necessary calculations 13 provide plots and graphs. Complexation wl diphenyl carbazide (OPC) in acidic media is another type of sensitive photometric method used for the analysis of aqueous. hexavalent chromium. At 540nm the cherry-red complex as a result of DPC reaction w/ chromium can be photometrically measured. at this wavelength. - a 25mL The UV-vis analysis for the determination of nexavalent chromium in ground water sample is given below. The experiment was based on external calibration method w/ each measurement sample prepared are as follows lab sample analysis contained the standard 100 ppb croy cor groundwater sample, volumes used as indicated below), 12.50 mL of 0.02 M H2Soy and 5.50 ml of 100 ppm DPC (wi water to adjust final volume to 25-ml). The main stripping method was square wave voltammetry, following the conditions set in the main ASV experiment. Standard 100 Volumetric Groundwater H2SO4 0.20 M, flask Sample, mL ppb CrO4*, 100…arrow_forwardplease helparrow_forward
- Predict the products of the following reactions. Draw mechanism arrows for each step for a, b, and c. a.) HBr b.) HI H₂O H2SO4 d.) C12 HO H2SO4 1.) BH3 2.) H2O2, NaOHarrow_forwardK for the following reaction is 0.11 at constant temperature. If the equilibrium concentration of HCl is 0.5 M, what is the equilibrium concentration of NH3. NH4CI(s) ⇌ NH3(g) + HCI(g)arrow_forwardplease help by Draw the following structures (Lewis or line-angle drawing).arrow_forward
- please helparrow_forwardConsider the reaction: 2 A (aq) ⇌ B(aq) Given the following KC values and starting with the initial concentration of A = 4.00 M, complete ICE diagram(s)and find the equilibrium concentrations for A and B.A) KC = 4.00B) KC = 200C) KC = 8.00 x10-3arrow_forward5) Consider the reaction: Cl2 (g) + F2 (g) ⟷ 2 ClF (g) KP=? The partial pressure of 203 kPa for Cl2 and a partial pressure of 405 kPa for F2. Upon reaching equilibrium, thepartial pressure of ClF is 180 kPa. Calculate the equilibrium concentrations and then find the value for KP.arrow_forward
- Don't used hand raiting and don't used Ai solutionarrow_forward(9 Pts) In one of the two Rare Earth element rows of the periodic table, identify an exception tothe general ionization energy (IE) trend. For the two elements involved, answer the followingquestions. Be sure to cite sources for all physical data that you use.a. (2 pts) Identify the two elements and write their electronic configurations.b. (2 pts) Based on their configurations, propose a reason for the IE trend exception.c. (5 pts) Calculate effective nuclear charges for the last electron in each element and theAllred-Rochow electronegativity values for the two elements. Can any of these valuesexplain the IE trend exception? Explain how (not) – include a description of how IErelates to electronegativity.arrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning
Linear Combination of Atomic Orbitals LCAO; Author: Edmerls;https://www.youtube.com/watch?v=nq1zwrAIr4c;License: Standard YouTube License, CC-BY
Quantum Molecular Orbital Theory (PChem Lecture: LCAO and gerade ungerade orbitals); Author: Prof Melko;https://www.youtube.com/watch?v=l59CGEstSGU;License: Standard YouTube License, CC-BY