
General Chemistry: Atoms First
2nd Edition
ISBN: 9780321809261
Author: John E. McMurry, Robert C. Fay
Publisher: Prentice Hall
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 1.28CP
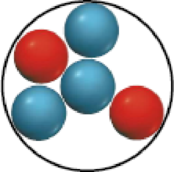
In the following drawings, red spheres represent protons and blue spheres represent neutrons. Which of the drawings represent different


Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.
What is the final product when D-galactose reacts with hydroxylamine?
Indicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.
Chapter 1 Solutions
General Chemistry: Atoms First
Ch. 1.1 - Look at the alphabetical list of elements inside...Ch. 1.1 - Prob. 1.2PCh. 1.3 - Identify the following elements as metals,...Ch. 1.3 - Prob. 1.4CPCh. 1.5 - Compounds A and B are colorless gases obtained by...Ch. 1.7 - The gold foil Rutherford used in his scattering...Ch. 1.7 - A small speck of carbon the size of a pinhead...Ch. 1.8 - The isotope S3475e is used medically for the...Ch. 1.8 - Chlorine, one of the elements in common table salt...Ch. 1.8 - An atom of element X contains 47 protons and 62...
Ch. 1.9 - Copper metal has two naturally occurring isotopes:...Ch. 1.9 - Based on your answer to Problem 1.11, how many...Ch. 1.9 - What is the mass in grams of each of the following...Ch. 1.9 - How many moles are in each of the following...Ch. 1.11 - Prob. 1.15PCh. 1.11 - Prob. 1.16PCh. 1.11 - Prob. 1.17CPCh. 1.11 - Prob. 1.18PCh. 1.11 - Prob. 1.19PCh. 1 - Prob. 1.20CPCh. 1 - Where on the following outline of a periodic table...Ch. 1 - Prob. 1.22CPCh. 1 - Prob. 1.23CPCh. 1 - If yellow spheres represent sulfur atoms and red...Ch. 1 - Prob. 1.25CPCh. 1 - Prob. 1.26CPCh. 1 - Prob. 1.27CPCh. 1 - In the following drawings, red spheres represent...Ch. 1 - Isotope A decays to isotope E through the...Ch. 1 - Prob. 1.30SPCh. 1 - Prob. 1.31SPCh. 1 - Prob. 1.32SPCh. 1 - Prob. 1.33SPCh. 1 - Prob. 1.34SPCh. 1 - Prob. 1.35SPCh. 1 - Prob. 1.36SPCh. 1 - Prob. 1.37SPCh. 1 - Prob. 1.38SPCh. 1 - Prob. 1.39SPCh. 1 - Prob. 1.40SPCh. 1 - Prob. 1.41SPCh. 1 - Prob. 1.42SPCh. 1 - Prob. 1.43SPCh. 1 - Prob. 1.44SPCh. 1 - Prob. 1.45SPCh. 1 - Prob. 1.46SPCh. 1 - Prob. 1.47SPCh. 1 - Prob. 1.48SPCh. 1 - Prob. 1.49SPCh. 1 - Prob. 1.50SPCh. 1 - Prob. 1.51SPCh. 1 - Prob. 1.52SPCh. 1 - Prob. 1.53SPCh. 1 - Prob. 1.54SPCh. 1 - Prob. 1.55SPCh. 1 - Prob. 1.56SPCh. 1 - Prob. 1.57SPCh. 1 - Prob. 1.58SPCh. 1 - Prob. 1.59SPCh. 1 - Prob. 1.60SPCh. 1 - If 6.02 1023 atoms of element Y have a mass of...Ch. 1 - Prob. 1.62SPCh. 1 - Prob. 1.63SPCh. 1 - Prob. 1.64SPCh. 1 - Prob. 1.65SPCh. 1 - Prob. 1.66SPCh. 1 - Prob. 1.67SPCh. 1 - Prob. 1.68SPCh. 1 - Prob. 1.69SPCh. 1 - Prob. 1.70SPCh. 1 - Prob. 1.71SPCh. 1 - Prob. 1.72SPCh. 1 - Prob. 1.73SPCh. 1 - Prob. 1.74SPCh. 1 - Prob. 1.75SPCh. 1 - Prob. 1.76SPCh. 1 - Prob. 1.77SPCh. 1 - Prob. 1.78SPCh. 1 - Prob. 1.79SPCh. 1 - Prob. 1.80SPCh. 1 - Prob. 1.81SPCh. 1 - Prob. 1.82SPCh. 1 - Which of the following isotope symbols cant be...Ch. 1 - Prob. 1.84SPCh. 1 - Naturally occurring silver consists of two...Ch. 1 - Magnesium has three naturally occurring isotopes:...Ch. 1 - Prob. 1.87SPCh. 1 - Prob. 1.88SPCh. 1 - Prob. 1.89SPCh. 1 - Prob. 1.90SPCh. 1 - Prob. 1.91SPCh. 1 - Prob. 1.92SPCh. 1 - Prob. 1.93SPCh. 1 - Prob. 1.94SPCh. 1 - Prob. 1.95SPCh. 1 - Prob. 1.96SPCh. 1 - Prob. 1.97SPCh. 1 - Prob. 1.98SPCh. 1 - Prob. 1.99SPCh. 1 - Prob. 1.100SPCh. 1 - Prob. 1.101SPCh. 1 - Prob. 1.102CHPCh. 1 - Prob. 1.103CHPCh. 1 - Prob. 1.104CHPCh. 1 - Prob. 1.105CHPCh. 1 - Prob. 1.106CHPCh. 1 - Prob. 1.107CHPCh. 1 - Prob. 1.108CHPCh. 1 - Prob. 1.109CHPCh. 1 - Prob. 1.110CHPCh. 1 - The mass percent of an element in a compound is...Ch. 1 - Prob. 1.112CHPCh. 1 - Prob. 1.113CHPCh. 1 - In an alternate universe, the smallest negatively...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forward
- er your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward5.arrow_forward6.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
The Bohr Model of the atom and Atomic Emission Spectra: Atomic Structure tutorial | Crash Chemistry; Author: Crash Chemistry Academy;https://www.youtube.com/watch?v=apuWi_Fbtys;License: Standard YouTube License, CC-BY