
Chemistry
9th Edition
ISBN: 9781133611097
Author: Steven S. Zumdahl
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 114CP
As part of a science project, you study traffic patterns in your city at an intersection in the middle of downtown. You set up a device that counts the ears passing through this intersection for a 24-hr period during a weekday. The graph of hourly traffic looks like this.
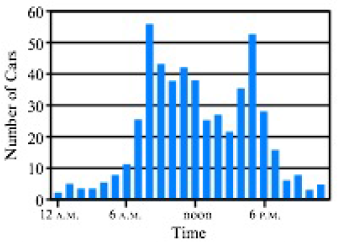
- a. At what time(s) does the highest number of cars pass through die intersection?
- b. At what time(s) does the lowest number of cars pass through die intersection?
- c. Briefly describe the trend in numbers of cars over the course of die day.
- d. Provide a hypothesis explaining the trend in numbers of cars over the course of the day.
- e. Provide a possible experiment that could test your hypothesis.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Predict the major products of the following organic reaction:
Some important notes:
Δ
CN
?
• Draw the major product, or products, of the reaction in the drawing area below.
• If there aren't any products, because no reaction will take place, check the box below the drawing area instead.
Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are
enantiomers.
ONO reaction.
Click and drag to start drawing a structure.
The following product was made from diethyl ketone and what other reagent(s)?
£
HO
10
2-pentyne
1-butyne and NaNH2
☐ 1-propanol
☐ pyridine
butanal
☐ pentanoate
Which pair of reagents will form the given product?
OH
X
+
Y
a.
CH3
b.
CH2CH3
༧་་
C. CH3-
CH2CH3
d.o6.(རི॰
e.
CH3
OCH2CH3
-MgBr
f. CH3-MgBr
g. CH3CH2-MgBr
-C-CH3
CH2CH3
Chapter 1 Solutions
Chemistry
Ch. 1 - Define and explain the differences between the...Ch. 1 - Is the scientific method suitable for solving...Ch. 1 - Which of the following statements could be tested...Ch. 1 - For each of the following pieces of glassware,...Ch. 1 - A student performed an analysis of a sample for...Ch. 1 - Compare and contrast the multiplication/division...Ch. 1 - Explain how density can be used as a conversion...Ch. 1 - On which temperature scale (F, C. or K) docs 1...Ch. 1 - Distinguish between physical changes and chemical...Ch. 1 - Why is the separation of mixtures into pure or...
Ch. 1 - a. There are 365 days per year, 24 hours per day,...Ch. 1 - Prob. 2ALQCh. 1 - When a marble is dropped into a beaker of water,...Ch. 1 - Prob. 4ALQCh. 1 - You may have noticed that when water boils, you...Ch. 1 - If you place a glass rod over a burning candle,...Ch. 1 - Which characteristics of a solid, a liquid, and a...Ch. 1 - Sketch a magnified view (showing atoms/molecules)...Ch. 1 - Paracelsus, a sixteenth-century alchemist and...Ch. 1 - What is wrong with the following statement? "The...Ch. 1 - Why is it incorrect to say that the results of a...Ch. 1 - You have a 1.0-cm3 sample of lead and a 1.0-cm3...Ch. 1 - Consider the addition of 15.4 to 28. What would a...Ch. 1 - Consider multiplying 26.2 by 16.43. What would a...Ch. 1 - The difference between a law and a theory is the...Ch. 1 - The scientific method is a dynamic process. What...Ch. 1 - Explain the fundamental steps of the scientific...Ch. 1 - Prob. 20QCh. 1 - A measurement is a quantitative observation...Ch. 1 - To determine the volume of a cube, a student...Ch. 1 - What are significant figures? Show how to indicate...Ch. 1 - A cold front moves through and the temperature...Ch. 1 - Give four examples illustrating each of the...Ch. 1 - Which of the following are exact numbers? a. There...Ch. 1 - Indicate the number of significant figures in each...Ch. 1 - How many significant figures are there in each of...Ch. 1 - How many significant figures are in each of the...Ch. 1 - Round off each of the following numbers to the...Ch. 1 - Use exponential notation to express the number...Ch. 1 - You have liquid in each graduated cylinder shown:...Ch. 1 - The beakers shown below have different precisions....Ch. 1 - Evaluate each of the following, and write the...Ch. 1 - Perform the following mathematical operations, and...Ch. 1 - Perform the following mathematical operations, and...Ch. 1 - Perform the following mathematical operations, and...Ch. 1 - Perform each of the following conversions. a. 8.43...Ch. 1 - a. How many kilograms are in 1 teragram? b. How...Ch. 1 - Perform the following unit conversions. a....Ch. 1 - Perform the following unit conversions. a. 908 oz...Ch. 1 - Use the following exact conversion factors to...Ch. 1 - Although the preferred SI unit of area is the...Ch. 1 - Precious metals and gems are measured in troy...Ch. 1 - Apothecaries (druggists) use the following set of...Ch. 1 - For a pharmacist dispensing pills or capsules, it...Ch. 1 - A children's pain relief elixir contains 80. mg...Ch. 1 - Science fiction often uses nautical analogies to...Ch. 1 - The world record for the hundred meter dash is...Ch. 1 - Would a car traveling at a constant speed of 65...Ch. 1 - You pass a road sign saying New York 112 km. If...Ch. 1 - Prob. 53ECh. 1 - In recent years, there has been a large push for...Ch. 1 - Prob. 55ECh. 1 - Carbon monoxide (CO) detectors sound an alarm when...Ch. 1 - Convert the following Fahrenheit temperatures to...Ch. 1 - A thermometer gives a reading of 96.1F 0.2F. What...Ch. 1 - Convert the following Celsius temperatures to...Ch. 1 - Convert the following Kelvin temperatures to...Ch. 1 - At what temperature is the temperature in degrees...Ch. 1 - The average daytime temperatures on the earth and...Ch. 1 - Use the figure below to answer the following...Ch. 1 - Ethylene glycol is the main component in...Ch. 1 - A material will float on the surface of a liquid...Ch. 1 - Prob. 66ECh. 1 - A star is estimated to have a mass of 2 1036 kg....Ch. 1 - A rectangular block has dimensions 2.9 cm 3.5 cm ...Ch. 1 - Diamonds are measured in carats, and 1 carat =...Ch. 1 - Ethanol and benzene dissolve in each other. When...Ch. 1 - A sample containing 33.42 g of metal pellets is...Ch. 1 - The density of pure silver is 10.5 g/cm3 at 20C....Ch. 1 - In e-ach of the following pairs, which has the...Ch. 1 - a. Calculate the mass of ethanol in 1.50 qt of...Ch. 1 - In each of the following pairs, which has the...Ch. 1 - Using Table 1.5, calculate the volume of 25.0 g of...Ch. 1 - The density of osmium (the densest metal) is 22.57...Ch. 1 - A copper wire (density = 8.96 g/cm3) has a...Ch. 1 - Match each description below with the following...Ch. 1 - Define the following terms: solid, liquid, gas,...Ch. 1 - What is the difference between homogeneous and...Ch. 1 - Classify the following mixtures as homogeneous or...Ch. 1 - Classify each of the following as a mixture or a...Ch. 1 - Suppose a teaspoon of magnesium filings and a...Ch. 1 - If a piece of hard, white blackboard chalk is...Ch. 1 - During a very cold winter, the temperature may...Ch. 1 - Classify the following as physical or chemical...Ch. 1 - The properties of a mixture are typically averages...Ch. 1 - In Shakespeares Richard III, the First Murderer...Ch. 1 - Prob. 91AECh. 1 - In the opening scenes of the movie Raiders of the...Ch. 1 - Prob. 93AECh. 1 - This year, like many past years, you begin to feel...Ch. 1 - Which of the following are chemical changes? Which...Ch. 1 - A column of liquid is found to expand linearly on...Ch. 1 - A 25.00-g sample of a solid is placed in a...Ch. 1 - For each of the following, decide which block is...Ch. 1 - According to the Official Rules of Baseball, a...Ch. 1 - The density of an irregularly shaped object was...Ch. 1 - The chemist in Example 1.14 did some further...Ch. 1 - The longest river in the world is the Nile River...Ch. 1 - Secretariat is known as the horse with the fastest...Ch. 1 - The hottest temperature recorded in the United...Ch. 1 - Prob. 106CWPCh. 1 - Which of the following statements is(are) true? a....Ch. 1 - Which of the following describes a chemical...Ch. 1 - A rule of thumb in designing experiments is to...Ch. 1 - Draw a picture showing the markings (graduations)...Ch. 1 - Many times errors are expressed in terms of...Ch. 1 - A person weighed 15 pennies on a balance and...Ch. 1 - On October 21, 1982, the Bureau of the Mint...Ch. 1 - As part of a science project, you study traffic...Ch. 1 - Sterling silver is a solid solution of silver and...Ch. 1 - Make molecular-level (microscopic) drawings for...Ch. 1 - Confronted with the box shown in the diagram, you...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Question 3 What best describes the product of the following reaction? 1. CH3CH2MgBr (2 eq) 2. H a new stereocenter will not be formed a new stereocenter will be formed an alkyl halide will result an alkane will result an aromatic compound will result 1 ptsarrow_forwardRank the following from most to least reactive toward nucleophilic attack. 1. [Select] [Select] 2. Acyl halide Aldehyde 3. Carboxylate ion 4. Carboxylic acid Ketone 5. [Select]arrow_forwardQuestion 10 1 pts Which of the following is the most accurate nomenclature? 1-hydroxy-1-methyldecane-4,7-dione 2-hydroxy-2-methyldecane-5,8-dione 4,6-dioxo-2-methyldecane-2-ol 9-hydroxy-9-methyldecane-3,6-dione 8-hydroxy-8-methylnonane-3,6-dione OHarrow_forward
- Could you please explain whether my thinking is correct or incorrect regarding how I solved it? Please point out any mistakes in detail, with illustrations if needed.arrow_forwardWhat are the most proper reagents to achieve these products? سد 1. 2. OH ○ 1. BrMgC6H6; 2. H+ ○ 1. BrMgCH2CH2CH2CH2CH3; 2. H+ O 1. CH3CH2CHO; 2. H+ O 1. BrMgCH2CH3; 2. H+arrow_forwardProvide the IUPAC (systematic) name only for the following compound. Dashes, commas, and spaces must be correct. Harrow_forward
- Please use the nernst equation to genereate the Ion Selective Electrode Analysis standard curve within my excel spread sheet. Nernst Equation: E = Eo + m (ln a) Link: https://mnscu-my.sharepoint.com/:x:/g/personal/vi2163ss_go_minnstate_edu/EaREe1-PfGNKq1Cbink6kkYB5lBy05hEaE3mbGPUb22S6w?rtime=zQaSX3xY3Ugarrow_forwarda) b) c) H NaOH heat, dehydration + KOH heat, dehydration NaOH + (CH3)3CCHO heat, dehydration Pharrow_forwardshow mechanismarrow_forward
- Please draw by handarrow_forward3. Predict the major product and give a mechanism for the following reactions: (CH3)3COH/H₂SO4 a) b) NC CH₂O c) LOCH, (CH3)3COH/H2SO4 H,SO -OHarrow_forwardIndicate if the aldehyde shown reacts with the provided nucleophiles in acid or base conditions. a NaBH4 be Li eli -NH2 P(Ph3) f KCN g OH excess h CH3OH i NaCHCCH3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning


General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning
Types of Matter: Elements, Compounds and Mixtures; Author: Professor Dave Explains;https://www.youtube.com/watch?v=dggHWvFJ8Xs;License: Standard YouTube License, CC-BY