Use the following scenario and results to answer the following four questions: Scenario: It was suspected that a particular drug sample contained three components: X, Y and Z. To check this, the mixture was analyzed by TLC with a nonpolar developing solvent on silica gel. The following TLC plate was obtained. Which components are most likely to be present in the sample? X and Z Y only Y and Z Y and another unknown component X and Y
Use the following scenario and results to answer the following four questions: Scenario: It was suspected that a particular drug sample contained three components: X, Y and Z. To check this, the mixture was analyzed by TLC with a nonpolar developing solvent on silica gel. The following TLC plate was obtained. Which components are most likely to be present in the sample? X and Z Y only Y and Z Y and another unknown component X and Y
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
Use the following scenario and results to answer the following four questions:
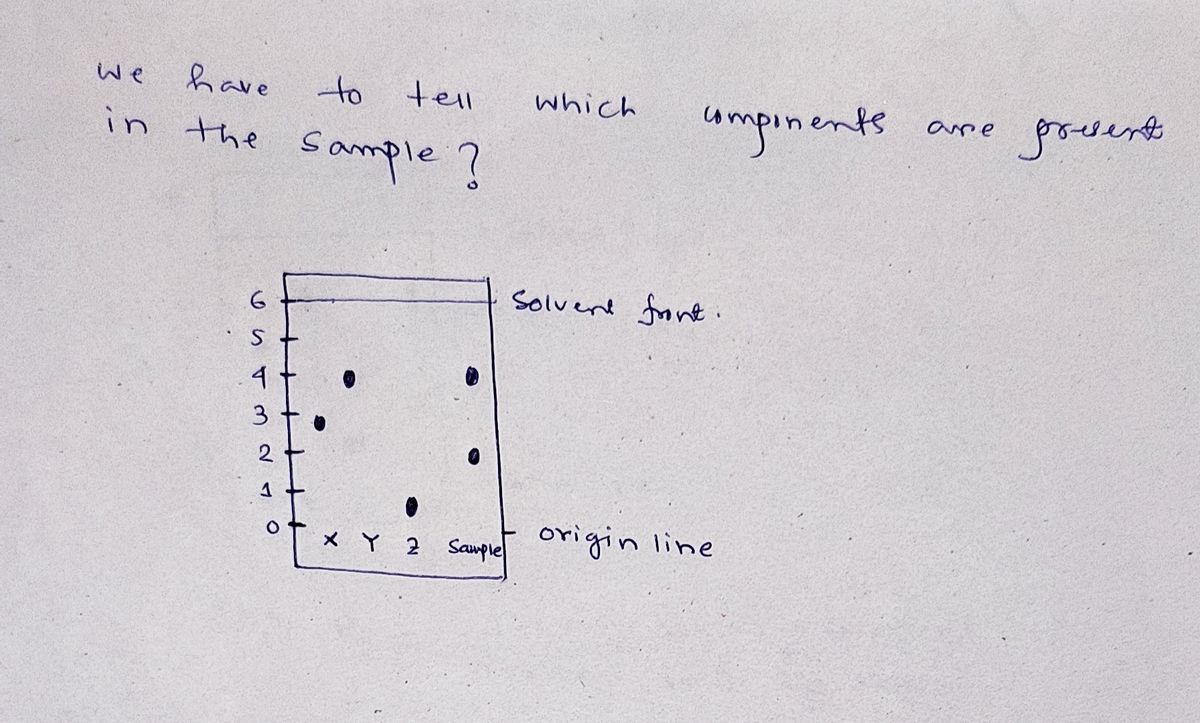
Scenario: It was suspected that a particular drug sample contained three components: X, Y and Z. To check this, the mixture was analyzed by TLC with a nonpolar developing solvent on silica gel. The following TLC plate was obtained.
Which components are most likely to be present in the sample?
|
X and Z |
||
|
Y only |
||
|
Y and Z |
||
|
Y and another unknown component |
||
|
X and Y |

Transcribed Image Text:### Paper Chromatography Experiment Illustration
#### Diagram Description:
The diagram consists of two panels labeled "before" and "after," each representing a stage in a paper chromatography process.
- **Before Panel:**
- Shows a horizontal baseline at the bottom of the paper labeled "origin line."
- Four dots aligned along this line are labeled X, Y, Z, and "sample" representing different substances or mixtures that are to be separated.
- **After Panel:**
- Includes the same vertical scale marked in centimeters from 0 to 6 cm, with 0 being at the origin line.
- The "solvent front" is drawn at the 6 cm mark, representing the furthest point the solvent has traveled.
- Dots corresponding to the substances have moved upwards to different heights:
- X has traveled to approximately 3 cm.
- Y has traveled to approximately 4.5 cm.
- Z has traveled to about 2.5 cm.
- "Sample" shows multiple spots, indicating it is a mixture.
### Explanation:
This diagram illustrates how paper chromatography is used to separate substances in a mixture. Each substance travels a different distance based on its affinity for the solvent versus the stationary phase of the paper. The positions of the spots after the process indicate the relative solubilities.
Expert Solution
Step 1
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY