how to interpret Saturation curves for hemoglobin and myoglobin and how these curves change when allosteric effectors are present.
Enzyme kinetics
In biochemistry, enzymes are proteins that act as biological catalysts. Catalysis is the addition of a catalyst to a chemical reaction to speed up the pace of the reaction. Catalysis can be categorized as either homogeneous or heterogeneous, depending on whether the catalysts are distributed in the same phase as that of the reactants. Enzymes are an essential part of the cell because, without them, many organic processes would slow down and thus will affect the processes that are important for cell survival and sustenance.
Regulation of Enzymes
A substance that acts as a catalyst to regulate the reaction rate in the living organism's metabolic pathways without itself getting altered is an enzyme. Most of the biological reactions and metabolic pathways in the living systems are carried out by enzymes. They are specific for their works and work in particular conditions. It maintains the best possible rate of reaction in the most stable state. The enzymes have distinct properties as they can proceed with the reaction in any direction, their particular binding sites, pH specificity, temperature specificity required in very few amounts.
how to interpret Saturation curves for hemoglobin and
myoglobin and how these curves change when allosteric effectors
are present.
An oxygen dissociation curve is obtained when we plot the %saturation of hemoglobin against the partial pressure of oxygen.
The oxygen dissociation curve represents the affinity and cooperative binding of oxygen to hemoglobin.
It looks like:
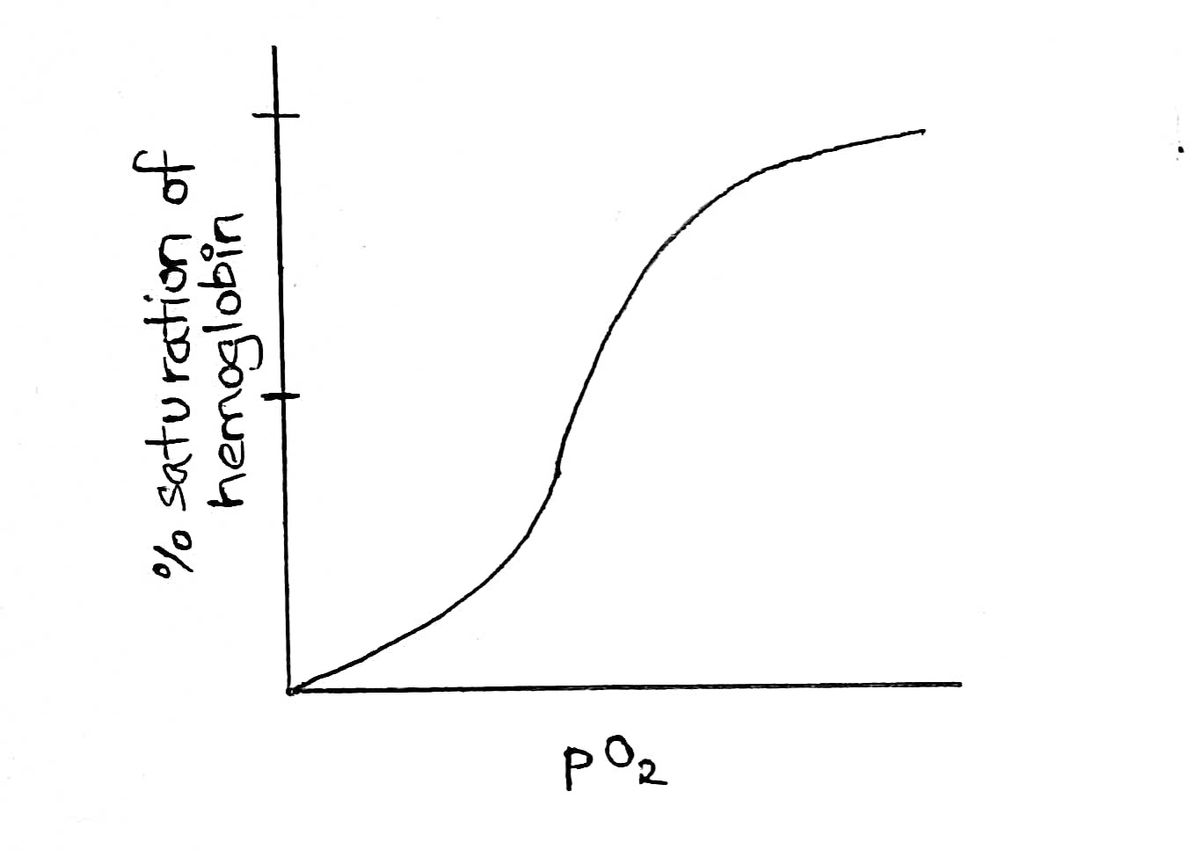
So we can see that the affinity for oxygen increases as the pO2 increases.
The curve is read by looking at the pO2 on x axis and corresponding % saturation value on the Y axis. If 50% saturation is obtained for smaller values of pO2 it means that hemoglobin has more affinity for oxygen. If 50% saturation is not obtained even after increasing the pO2, then hemoglobin has less affinity for oxygen.
Step by step
Solved in 2 steps with 2 images









