A clinical trial is conducted to compare an experimental medication to placebo to reduce the symptoms of asthma. One hundred participants are enrolled in the study and randomized to receive either the experimental medication or placebo. The primary outcome is self- reported a reduction of symptoms. Among 50 participants who received the experimental medication., 30 report a reduction of symptoms as compared to 15 participants of 50 assigned to the placebo. Generate a 95% CI for the difference in proportions of participants reporting a reduction of symptoms between the experimental and placebo groups.
A clinical trial is conducted to compare an experimental medication to placebo to reduce the symptoms of asthma. One hundred participants are enrolled in the study and randomized to receive either the experimental medication or placebo. The primary outcome is self- reported a reduction of symptoms. Among 50 participants who received the experimental medication., 30 report a reduction of symptoms as compared to 15 participants of 50 assigned to the placebo. Generate a 95% CI for the difference in proportions of participants reporting a reduction of symptoms between the experimental and placebo groups.
Given information can be formulated in the table showing the numbers based on the study given below :
The table is as follows :
| Reduction | No Reduction | Total | |
| Medication | 30 | 20 | 50 |
| Placebo | 15 | 35 | 50 |
Relative risk can be calculated :
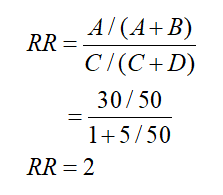
Assuming the reduction of of symptoms as incidence in each of the medication and placebo groups .
Step by step
Solved in 2 steps with 1 images









