3b) please see attached


The given balanced reaction is:
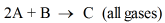
Suppose the rate law for above reaction is,
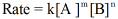
here, k is the rate constant,
m and n are the order of reaction with respect to the reactants A and B respectively.
The values for m and n are calculated as follows,
By using the given table, the initial [A] is constant in experiment 1 and 3. Thus the rate aw expressions for experiment 1 and 3 are written as,
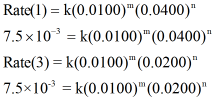
Now, dividing the rate law expressions for reaction 1 and 3 we can get value of n as shown below,
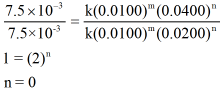
As the value of n is zero, the order of reaction with respect to reactant B is zero.
Now, in the given table, the initial [B] is constant in experiment 2 and 3. Thus the rate aw expressions for experiment 2 and 3 are written as,
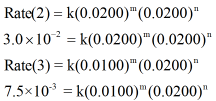
Now, dividing the rate law expressions for reaction 2 and 3 we can get value of m as shown below,
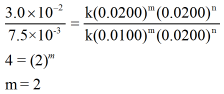
As the value of m = 2, the order of reaction with respect to reactant A is 2.
Step by step
Solved in 3 steps with 8 images









