
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter NW1, Problem 6CTQ
Interpretation Introduction
Interpretation: The alternate seven-carbon parent chain in below heptane should be indicated.
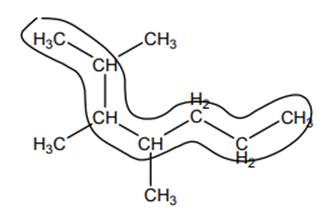
Concept introduction: The IUPAC name begins with prefix to designate the number of
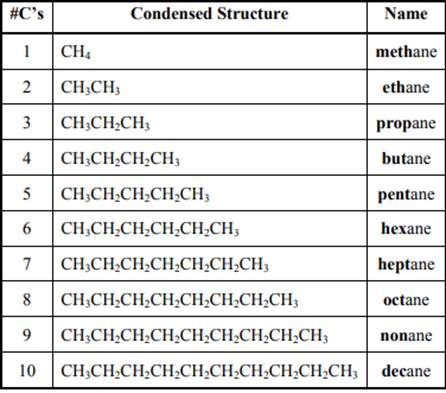
The IUPAC system for nomenclature of straight hydrocarbon makes use of table given as follows:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
For the decomposition reaction of N2O5(g): 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed:
N2O5 NO2 + NO3 (K1) | NO2 + NO3 → N2O5 (k-1) | NO2 + NO3 NO2 + O2 + NO (k2) | NO + N2O51 NO2 + NO2 + NO2 (K3)
→
Give the expression for the acceptable rate.
→
→
(A).
d[N205]
dt
==
2k,k₂[N₂O₂]
k₁+k₁₂
(B).
d[N2O5]
=-k₁[N₂O] + k₁[NO₂] [NO3] - k₂[NO₂]³
dt
(C).
d[N2O5]
=-k₁[N₂O] + k [NO] - k₂[NO] [NO]
d[N2O5]
(D).
=
dt
= -k₁[N2O5] - k¸[NO][N₂05]
dt
Do not apply the calculations, based on the approximation of the stationary state, to make them perform correctly. Basta discard
the 3 responses that you encounter that are obviously erroneous if you apply the formula to determine the speed of a reaction.
For the decomposition reaction of N2O5(g): 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed:
N2O5 NO2 + NO3 (K1) | NO2 + NO3 → N2O5 (k-1) | NO2 + NO3 NO2 + O2 + NO (k2) | NO + N2O51 NO2 + NO2 + NO2 (K3)
→
Give the expression for the acceptable rate.
→
→
(A).
d[N205]
dt
==
2k,k₂[N₂O₂]
k₁+k₁₂
(B).
d[N2O5]
=-k₁[N₂O] + k₁[NO₂] [NO3] - k₂[NO₂]³
dt
(C).
d[N2O5]
=-k₁[N₂O] + k [NO] - k₂[NO] [NO]
d[N2O5]
(D).
=
dt
= -k₁[N2O5] - k¸[NO][N₂05]
dt
Do not apply the calculations, based on the approximation of the stationary state, to make them perform correctly. Basta discard
the 3 responses that you encounter that are obviously erroneous if you apply the formula to determine the speed of a reaction.
R lactam or lactone considering as weak acid or weak base and why
Chapter NW1 Solutions
Organic Chemistry: A Guided Inquiry
Ch. NW1 - Prob. 1CTQCh. NW1 - (E) Write a correct name below each of the...Ch. NW1 - (E) What suffix do all the names in Model 1 have...Ch. NW1 - (E) What prefix stands for eight carbons?Ch. NW1 - Prob. 5CTQCh. NW1 - Prob. 6CTQCh. NW1 - Prob. 7CTQCh. NW1 - Prob. 8CTQCh. NW1 - Prob. 9CTQCh. NW1 - Use Model 1 to propose names for three-, four-,...
Ch. NW1 - Prob. 11CTQCh. NW1 - Prob. 12CTQCh. NW1 - Prob. 13CTQCh. NW1 - Name the following alkanes.Ch. NW1 - (Check your work.) Explain what is wrong with each...Ch. NW1 - Prob. 16CTQCh. NW1 - Draw the following alkanes a....Ch. NW1 - Prob. 18CTQCh. NW1 - Draw structures that correspond to the following...Ch. NW1 - For mono-substituted cycloalkanes the “1” is not...Ch. NW1 - Prob. 21CTQCh. NW1 - Prob. 22CTQCh. NW1 - Prob. 23CTQCh. NW1 - Prob. 24CTQCh. NW1 - Prob. 25CTQCh. NW1 - Write the name of the molecule on the left using...Ch. NW1 - Prob. 1ECh. NW1 - Prob. 2ECh. NW1 - Name each of the following structures.
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 81. a. Propose a mechanism for the following reaction: OH CH2=CHCHC=N b. What is the product of the following reaction? HO H₂O N=CCH2CH2CH OH HO CH3CCH=CH2 H₂O C=N 82. Unlike a phosphonium ylide that reacts with an aldehyde or a ketone to form an alkene a sulfonium uliaarrow_forwardFor each reaction below, decide if the first stable organic product that forms in solution will create a new CC bond, and check the appropriate box. Next, for each reaction to which you answered "Yes" to in the table, draw this product in the drawing area below. Note for advanced students: for this problem, don't worry if you think this product will continue to react under the current conditions - just focus on the first stable product you expect to form in solution. ? NH2 MgBr Will the first product that forms in this reaction create a new CC bond? ○ Yes ○ No MgBr ? Will the first product that forms in this reaction create a new CC bond? O Yes O No Click and drag to start drawing a structure. :☐ G x c olo Ar HEarrow_forwardPredicting As the lead product manager at OrganometALEKS Industries, you are trying to decide if the following reaction will make a molecule with a new C - C bond as its major product: H₂N O H 1. ? 2. H3O+ If this reaction will work, draw the major organic product or products you would expect in the drawing area below. If there's more than one major product, you can draw them in any arrangement you like. Be sure you use wedge and dash bonds if necessary, for example to distinguish between major products with different stereochemistry. 0 If the major products of this reaction won't have a new CC bond, just check the box under the drawing area and leave it blank. فا Explanation Check Click and drag to start drawing a structure.arrow_forward
- Highlight the chirality (or stereogenic) center(s) in the given compound. A compound may have one or more stereogenic centers. OH OH OH OH OH OHarrow_forwardUsing wedge-and-dash bonds, modify the bonds on the chiral carbon in the molecule below so the molecule has R stereochemical configuration. NH H Br X टेarrow_forwardProvide photos of models of the following molecules. (Include a key for identification of the atoms) 1,2-dichloropropane 2,3,3-trimethylhexane 2-bromo-3-methybutanearrow_forward
- Please draw the structure in the box that is consistent with all the spectral data and alphabetically label all of the equivalent protons in the structure (Ha, Hb, Hc....) in order to assign all the proton NMR peaks. The integrations are computer generated and approximate the number of equivalent protons. Molecular formula: C13H1802 14 13 12 11 10 11 (ppm) Structure with assigned H peaks 2.08 3.13arrow_forwardA 0.10 M solution of acetic acid (CH3COOH, Ka = 1.8 x 10^-5) is titrated with a 0.0250 M solution of magnesium hydroxide (Mg(OH)2). If 10.0 mL of the acid solution is titrated with 10.0 mL of the base solution, what is the pH of the resulting solution?arrow_forwardFirefly luciferin exhibits three rings. Identify which of the rings are aromatic. Identify which lone pairs are involved in establishing aromaticity. The lone pairs are labeled A-D below.arrow_forward
- A 0.10 M solution of acetic acid (CH3COOH, Ka = 1.8 x 10^-5) is titrated with a 0.0250 M solution of magnesium hydroxide (Mg(OH)2). If 10.0 mL of the acid solution is titrated with 10.0 mL of the base solution, what is the pH of the resulting solution?arrow_forwardGiven a complex reaction with rate equation v = k1[A] + k2[A]2, what is the overall reaction order?arrow_forwardPlease draw the structure in the box that is consistent with all the spectral data and alphabetically label all of the equivalent protons in the structure (Ha, Hb, Hc....) in order to assign all the proton NMR peaks. The integrations are computer generated and approximate the number of equivalent protons. Molecular formula: C13H1802 14 13 12 11 10 11 (ppm) Structure with assigned H peaks 2.08 3.13arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co