
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter NW1, Problem 11CTQ
Interpretation Introduction
Interpretation: The information presented by numbers written in parent names in below figure should be given.
Concept introduction: The IUPAC name begins with prefix to designate the number of
The IUPAC system for nomenclature of straight hydrocarbon makes use of table given as follows:
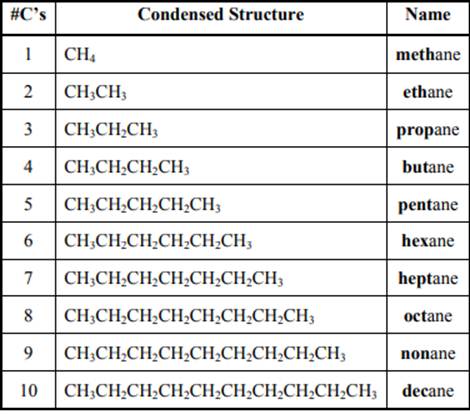
As per IUPAC recommendations, longest chain found in a continuous manner in a branched molecule is chosen as parent chain. All the side chains are named in alphabetical order.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw the major product of this reaction. Ignore inorganic byproducts.
Assume that the water side product is continuously removed to drive the reaction toward products.
O
CH3CH2NH2, TSOH
Select to Draw
>
Predict the major organic product(s) for the following reaction.
Predict the major organic product(s) for the following reactions.
Chapter NW1 Solutions
Organic Chemistry: A Guided Inquiry
Ch. NW1 - Prob. 1CTQCh. NW1 - (E) Write a correct name below each of the...Ch. NW1 - (E) What suffix do all the names in Model 1 have...Ch. NW1 - (E) What prefix stands for eight carbons?Ch. NW1 - Prob. 5CTQCh. NW1 - Prob. 6CTQCh. NW1 - Prob. 7CTQCh. NW1 - Prob. 8CTQCh. NW1 - Prob. 9CTQCh. NW1 - Use Model 1 to propose names for three-, four-,...
Ch. NW1 - Prob. 11CTQCh. NW1 - Prob. 12CTQCh. NW1 - Prob. 13CTQCh. NW1 - Name the following alkanes.Ch. NW1 - (Check your work.) Explain what is wrong with each...Ch. NW1 - Prob. 16CTQCh. NW1 - Draw the following alkanes a....Ch. NW1 - Prob. 18CTQCh. NW1 - Draw structures that correspond to the following...Ch. NW1 - For mono-substituted cycloalkanes the “1” is not...Ch. NW1 - Prob. 21CTQCh. NW1 - Prob. 22CTQCh. NW1 - Prob. 23CTQCh. NW1 - Prob. 24CTQCh. NW1 - Prob. 25CTQCh. NW1 - Write the name of the molecule on the left using...Ch. NW1 - Prob. 1ECh. NW1 - Prob. 2ECh. NW1 - Name each of the following structures.
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Provide the complete mechanism for the reactions below. You must include appropriate arrows,intermediates, and formal charges.arrow_forwardIndicate the products obtained by reacting fluorobenzene with a sulfonitric mixture.arrow_forwardIf I have 1-bromopropene, to obtain compound A, I have to add NaOH and another compound. Indicate which compound that would be. C6H5 CH3arrow_forward
- If I have 1-bromopropene and I want to obtain (1,1-dipropoxyethyl)benzene, indicate the compound that I should add in addition to NaOH.arrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Ο HSCH2CH2CH2SH, BF3 Select to Draw I Submitarrow_forwardFeedback (7/10) Draw the major product of this reaction. Ignore inorganic byproducts. Assume that the water side product is continuously removed to drive the reaction toward products. Incorrect, 3 attempts remaining Ο (CH3CH2)2NH, TSOH Select to Draw V N. 87% Retryarrow_forward
- If I want to obtain (1,1-dipropoxyethyl)benzene from 1-bromopropene, indicate the product that I have to add in addition to NaOH.arrow_forwardIndicate the products obtained when fluorobenzene reacts with a sulfonitric acid mixture (HNO3 + H2SO4). Indicate the majority if necessary.arrow_forwardIndicate the products obtained when chlorobenzene acid reacts with a sulfonitric acid mixture (HNO3 + H2SO4). Indicate the majority if necessary.arrow_forward
- Indicate the products obtained by reacting benzenesulfonic acid with a sulfonitric acid mixture (HNO3 + H2SO4). Indicate the majority if necessary.arrow_forwardIndicate the products obtained by reacting ethylbenzene with a sulfonitric acid mixture (HNO3 + H2SO4). Indicate the majority if necessary.arrow_forwardIndicate the products obtained when tert-butylbenzene reacts with a sulfonitric acid mixture (HNO3 + H2SO4). Indicate the majority if necessary.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning