
(a)
Interpretation:
The structure of the molecule that corresponds to the given IUPAC name is to be drawn.
Concept introduction:
The rules for naming acid anhydrides are derived from the fact that an acid anhydride can be produced from two carboxylic acids in the dehydration reaction as shown below:
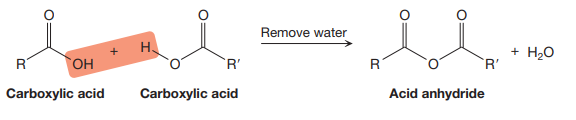
If the two R and R’ groups attached to the acid anhydride are the same, then the anhydride is symmetrical, but if they are different, the acid anhydride is unsymmetrical.
Symmetrical anhydrides are named according to the general form alkanoic anhydride in which the alkanoic portion corresponds to the specific
Unsymmetrical anhydrides are named according to the general form alkanoic alkanoic anhydride, where each alkanoic portion corresponds to different carboxylic acids that would be required to produce the anhydride. The two carboxylic acids follow the alphabetical order.
(b)
Interpretation:
The structure of the molecule that corresponds to the given IUPAC name is to be drawn.
Concept introduction:
The rules for naming acid anhydrides are derived from the fact that an acid anhydride can be produced from two carboxylic acids in the dehydration reaction as shown below:

If the two R and R’ groups attached to the acid anhydride are the same, then the anhydride is symmetrical, but if they are different, the acid anhydride is unsymmetrical.
Symmetrical anhydrides are named according to general form alkanoic anhydride where the alkanoic portion corresponds to the specific carboxylic acid that could undergo dehydration to produce the anhydride.
Unsymmetrical anhydrides are named according to the general form alkanoic alkanoic anhydride, where each alkanoic portion corresponds to different carboxylic acids that would be required to produce the anhydride. The two carboxylic acids follow the alphabetical order.
(c)
Interpretation:
The structure of the molecule is to be drawn that corresponds to the given IUPAC name.
Concept introduction:
The rules for naming acid anhydrides are derived from the fact that an acid anhydride can be produced from two carboxylic acids in the dehydration reaction as shown below:

If the two R and R’ groups attached to the acid anhydride are same, then the anhydride is symmetrical, but if they are different, the acid anhydride is unsymmetrical.
Symmetrical anhydride are named according to general form alkanoic anhydride where the alkanoic portion corresponds to the specific carboxylic acid that could undergo dehydration to produce the anhydride.
Unsymmetrical anhydrides are named according to the general form alkanoic alkanoic anhydride, where each alkanoic portion corresponds to different carboxylic acids that would be required to produce the anhydride. The two carboxylic acids follow the alphabetical order.
Substituents attached to the carbon chain of any carboxylic acid portion are written as prefix in the IUPAC name.
(d)
Interpretation:
The structure of the molecule is to be drawn that corresponds to the given IUPAC name.
Concept introduction:
The rules for naming acid anhydrides are derived from the fact that an acid anhydride can be produced from two carboxylic acids in the dehydration reaction as shown below:

If the two R and R’ groups attached to the acid anhydride are same, then the anhydride is symmetrical, but if they are different, the acid anhydride is unsymmetrical.
Symmetrical anhydride are named according to general form alkanoic anhydride where the alkanoic portion corresponds to the specific carboxylic acid that could undergo dehydration to produce the anhydride.
Unsymmetrical anhydrides are named according to the general form alkanoic alkanoic anhydride, where each alkanoic portion corresponds to different carboxylic acids that would be required to produce the anhydride. The two carboxylic acids follow the alphabetical order.
Substituents attached to the carbon chain of any carboxylic acid portion are written as prefix in the IUPAC name.
Want to see the full answer?
Check out a sample textbook solution
Chapter F Solutions
Organic Chemistry: Principles And Mechanisms: Study Guide/solutions Manual (second)
- CHEM2323 E Tt PS CH03 Draw and name all monobromo derivatives of pentane, C5H11Br. Problem 3-33 Name: Draw structures for the following: (a) 2-Methylheptane (d) 2,4,4-Trimethylheptane Problem 3-35 (b) 4-Ethyl-2,2-dimethylhexane (e) 3,3-Diethyl-2,5-dimethylnonane (c) 4-Ethyl-3,4-dimethyloctane 2 (f) 4-Isopropyl-3-methylheptane KNIE>arrow_forwardProblem 3-42 Consider 2-methylbutane (isopentane). Sighting along the C2-C3 bond: (a) Draw a Newman projection of the most stable conformation. (b) Draw a Newman projection of the least stable conformation. Problem 3-44 Construct a qualitative potential-energy diagram for rotation about the C-C bond of 1,2-dibromoethane. Which conformation would you expect to be most stable? Label the anti and gauche conformations of 1,2- dibromoethane. Problem 3-45 Which conformation of 1,2-dibromoethane (Problem 3-44) would you expect to have the largest dipole moment? The observed dipole moment of 1,2-dibromoethane is µ = 1.0 D. What does this tell you about the actual conformation of the molecule?arrow_forwardGas Law Studies 1. Mass of zinc Determination of 0.899 2) Moles of zinc 0.01361 mol 3.) Moles of hydrogen 00? ← I was told to calculate this number from mole of zinc. 350m So does that mean it will be 0.01361 mol too? 4 Volume of water collected (mL) 5) VL of water collected (Liters) 0.350 L 6) Temp of water collected (°C) 7) Temp of water collected (°K) 8) Atmospheric pressure (mm) 9) Vapor pressure of water (mm) 10) Corrected pressure of hydrogen 20% 29°C 764.0mm Hg (mm) 17.5mm 11) Corrected pressure of hydrogen (atm) 12) Experimentally calculated value of 19 13. Literature value of R 14) % Error 15) Suggest reasons for the % error (#14)arrow_forward
- No wedge or dashes. Do proper structure. Provide steps and explanation.arrow_forward10 Question (1 point) Draw curved arrow notation to indicate the proton transfer between NaOH and CH3CO₂H. 2nd attempt :0- H See Periodic Table See Hint Draw the products of the proton transfer reaction. Don't add a + sign between the products.arrow_forwardProvide steps and explanation please.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





