
Concept explainers
(a)
Interpretation:
The IUPAC name for the given compound is to be determined.
Concept introduction:
For a molecule that has more than one double bond or triple bond, the IUPAC name must indicate the number of double bonds or triple bonds present as well as their locations. To name the molecule with multiple double/triple bonds, establish the root as the longest carbon chain or the largest carbon ring that contains the greatest number of entire
Answer to Problem B.32P
The IUPAC name for the given compound is
Explanation of Solution
The given molecule is
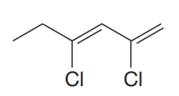
In the given molecule, the longest carbon chain containing all
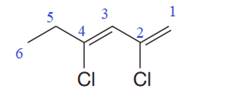
At carbon atoms C2 and C4 of the root, two chlorine atoms are present. Thus, the complete IUPAC name of this molecule is
The IUPAC name of the compound is written according to the rules for nomenclature.
(b)
Interpretation:
The IUPAC name for the given compound is to be determined.
Concept introduction:
For a molecule that has more than one double bond or triple bond, the IUPAC name must indicate the number of double bonds or triple bonds present as well as their locations. To name the molecule with multiple double/triple bonds, establish the root as the longest carbon chain or the largest carbon ring that contains the greatest number of entire
Answer to Problem B.32P
The IUPAC name for the given compound is
Explanation of Solution
The given compound is
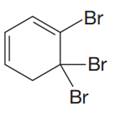
In the given molecule, the largest carbon ring containing all two
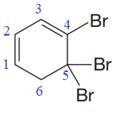
Three bromine atoms are present as substituents at carbon atoms C4, C5, and C5 to the root. The prefix ‘tri’ must be used to indicate the number of substituents present to the root. Thus, the complete IUPAC name of this molecule is
The IUPAC name of the compound is written according to the rules for nomenclature.
(c)
Interpretation:
The IUPAC name for the given compound is to be determined.
Concept introduction:
For a molecule that has more than one double bond or triple bond, the IUPAC name must indicate the number of double bonds or triple bonds present as well as their locations. To name the molecule with multiple double/triple bonds, establish the root as the longest carbon chain or the largest carbon ring that contains the greatest number of entire
Answer to Problem B.32P
The IUPAC name for the given compound is
Explanation of Solution
The given molecule is
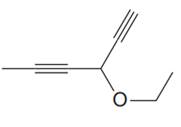
In the given molecule, the longest carbon chain containing all two
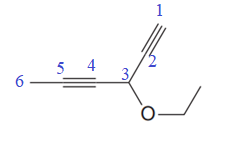
One methoxy substituent is attached to the C3 carbon atom of the root. Thus, the complete IUPAC name of this molecule is
The IUPAC name of the compound is written according to the rules for nomenclature.
(d)
Interpretation:
The IUPAC name for the given compound is to be determined.
Concept introduction:
For a molecule that has more than one double bond or triple bond, the IUPAC name must indicate the number of double bonds or triple bonds present as well as their locations. To name the molecule with multiple double/triple bonds, establish the root as the longest carbon chain or the largest carbon ring that contains the greatest number of entire
Answer to Problem B.32P
The IUPAC name for the given compound is
Explanation of Solution
The given molecule is
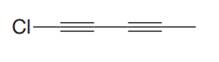
In the given molecule, the longest carbon chain containing all
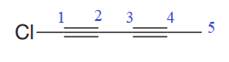
At carbon atom C1of the root, one chlorine atom is present.
Thus, the complete IUPAC name of this molecule is
The IUPAC name of the compound is written according to the rules for nomenclature.
(e)
Interpretation:
The IUPAC name for the given compound is to be determined.
Concept introduction:
For a molecule that has more than one double bond or triple bond, the IUPAC name must indicate the number of double bonds or triple bonds present as well as their locations. To name the molecule with multiple double/triple bonds, establish the root as the longest carbon chain or the largest carbon ring that contains the greatest number of entire
Answer to Problem B.32P
The IUPAC name for the given compound is
Explanation of Solution
The given compound is
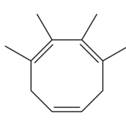
In the given molecule, the largest carbon ring containing all three
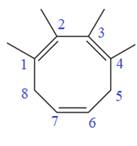
Four methyl substituents are attached at C1, C2, C3, and C4 carbon atoms of the root. The prefix ‘tetra’ should be used to indicate four methyl substituents.
Thus, the complete IUPAC name of this molecule is
The IUPAC name of the compound is written according to the rules for nomenclature.
(f)
Interpretation:
The IUPAC name for the given compound is to be determined.
Concept introduction:
For a molecule that has more than one double bond or triple bond, the IUPAC name must indicate the number of double bonds or triple bonds present as well as their locations. To name the molecule with multiple double/triple bonds, establish the root as the longest carbon chain or the largest carbon ring that contains the greatest number of entire
Answer to Problem B.32P
The IUPAC name for the given compound is
Explanation of Solution
The given molecule is
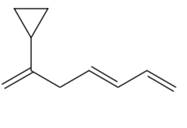
In the given molecule, the longest carbon chain containing all
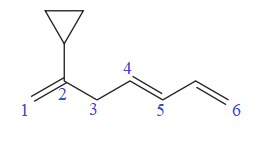
At carbon atom C2 of the root, one cyclopropyl group is attached.
Thus, the complete IUPAC name of this molecule is
The IUPAC name of the compound is written according to the rules for nomenclature.
(g)
Interpretation:
The IUPAC name for the given compound is to be determined.
Concept introduction:
For a molecule that has more than one double bond or triple bond, the IUPAC name must indicate the number of double bonds or triple bonds present as well as their locations. To name the molecule with multiple double/triple bonds, establish the root as the longest carbon chain or the largest carbon ring that contains the greatest number of entire
Answer to Problem B.32P
The IUPAC name for the given compound is
Explanation of Solution
The given molecule is
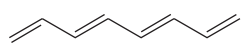
In the given molecule, the longest carbon chain containing all
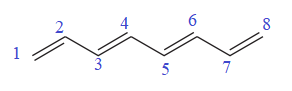
There are no substituents attached to the root. Thus, the complete IUPAC name of this molecule is
The IUPAC name of the compound is written according to the rules for nomenclature.
Want to see more full solutions like this?
Chapter B Solutions
Organic Chemistry: Principles And Mechanisms: Study Guide/solutions Manual (second)
- Complete the mechanismarrow_forward8 00 6 = 10 10 Decide whether each of the molecules in the table below is stable, in the exact form in which it is drawn, at pH = 11. If you decide at least one molecule is not stable, then redraw one of the unstable molecules in its stable form below the table. (If more than unstable, you can pick any of them to redraw.) Check OH stable HO stable Ounstable unstable O OH stable unstable OH 80 F6 F5 stable Ounstable X Save For Later Sub 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy C ཀྭ་ A F7 매 F8 F9 4 F10arrow_forwardJust try completing it and it should be straightforward according to the professor and TAs.arrow_forward
- The grading is not on correctness, so if you can just get to the correct answers without perfectionism that would be great. They care about the steps and reasoning and that you did something. I asked for an extension, but was denied the extension.arrow_forwardShow your work and do something that is reasonable. It does not have to be 100% correct. Just show something that looks good or pretty good as acceptable answers. Something that looks reasonable or correct would be sufficient. If you can get many of them correct that would be great!arrow_forwardShow your work and do something that is reasonable. It does not have to be 100% correct. Just show something that looks good or pretty good as acceptable answers. Something that looks reasonable or correct would be sufficient. If you can get many of them correct that would be great!arrow_forward
- Take a look at the following molecule, and then answer the questions in the table below it. (You can click the other tab to see the molecule without the colored regions.) with colored region plain 0= CH2-0-C-(CH2)16-CH3 =0 CH-O-C (CH2)7-CH=CH-(CH2)5-CH3 D CH3 | + OMPLO CH3-N-CH2-CH2-0-P-O-CH2 B CH3 A Try again * 000 Ar 8 0 ?arrow_forwardShow your work and do something that is reasonable. It does not have to be 100% correct. Just show something that looks good or pretty good as acceptable answers.arrow_forwardShow your work and do something that is reasonable. It does not have to be 100% correct. Just show something that looks good or pretty good as acceptable answers.arrow_forward
- = 1 = 2 3 4 5 6 ✓ 7 8 ✓ 9 =10 Devise a synthesis to prepare the product from the given starting material. Complete the following reaction scheme. Part 1 of 3 -Br Draw the structure for compound A. Check Step 1 Step 2 A Click and drag to start drawing a structure. × ↓m + OH Save For Later S 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privaarrow_forwardPredict the products of this organic reduction: 田 Check AP + + H2 Lindlar catalyst Click an drawing 2025 McGraw Hill LLC. All Rigarrow_forward70 Suppose the molecule below is in acidic aqueous solution. Is keto-enol tautomerization possible? • If a keto-enol tautomerization is possible, draw the mechanism for it. Be sure any extra reagents you add to the left-hand sid available in this solution. • If a keto-enol tautomerization is not possible, check the box under the drawing area. : ☐ Add/Remove step Click and drag to st drawing a structure Check Save For Late. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Usearrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

