
a)

Interpretation:
How to convert 1-butyne in to butanone is to be shown.
Concept introduction:
Terminal
To show:
How to convert 1-butyne in to butanone.
b)
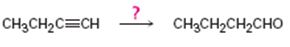
Interpretation:
How to convert 1-butyne in to butanal is to be shown.
Concept introduction:
Terminal alkynes when hydrated using hydroboration-oxidation reaction yield an enol with OH group attached to the terminal carbon as the reaction follows anti-Markovnikov regiochemistry. The enol obtained then tautomerizes to an
To show:
How to convert 1-butyne in to butanal is to be shown.
c)
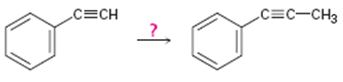
Interpretation:
How to convert ethynylbenzene in to propynylbenzene is to be shown.
Concept introduction:
Terminal alkynes are converted in to acetylides by treating with NaNH2 in liquid ammonia. The acetylides can be converted to higher alkynes by treating with
To show:
How to convert ethynylbenzene in to propynylbenzene.
d)
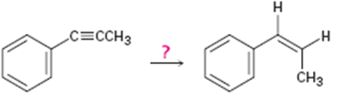
Interpretation:
How to convert propynylbenzene in to cis-1-propenylbenzene is to be shown.
Concept introduction:
Alkynes can be converted in to the corresponding
To show:
How to convert 1-propynylbenzene in to 1-propenylbenzene.
e)

Interpretation:
How to convert 1-butyne in to propanoic acid is to be shown.
Concept introduction:
Alkynes gets cleaved when treated with powerful oxidizing agents like KMnO4. Internal alkynes give carboxylic acids as products. Terminal alkynes give CO2 also along with a
To show:
How to convert 1-butyne in to propanoic acid.
f)
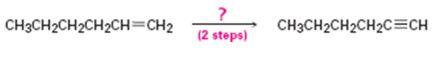
Interpretation:
How to convert 1-hexene in to 1-hexyne in two steps is to be shown.
Concept introduction:
Alkenes when treated with bromine yield a dibromo
To show:
How to convert 1-hexene in to 1-hexyne in two steps
Trending nowThis is a popular solution!

Chapter 9 Solutions
Bundle: Organic Chemistry, 9th, Loose-Leaf + OWLv2, 4 terms (24 months) Printed Access Card
- When anisole is treated with excess bromine, the reaction gives a product which shows two singlets in 1H NMR. Draw the product.arrow_forward(ii) Draw a reasonable mechanism for the following reaction: CI NaOH heat OH (hint: SNAr Reaction) :arrow_forwardDraw the major product in each of the following reaction:arrow_forward
- Draw the mechanism for the following Friedel-Craft reaction. AlBr3 Brarrow_forward(a) Draw the structures of A and B in the following reaction. (i) NaNH2, NH3(1) A + B (ii) H3O+arrow_forwardFor the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 →> NO₂+ NO3_(K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → → NO2 + O2 + NO (K2) NO + N2O5- NO2 + NO2 + NO2 (K3) d[N₂O5] __2k‚k₂[N2O5] Indicate whether the following rate expression is acceptable: dt k₁₁+ k₂arrow_forward
- Consider the following decomposition reaction of N2O5(g): For the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 → NO2 + NO3 (K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → NO2 + O2 + NO (K2) NO + N2O5 → NO2 + NO2 + NO2 (K3) Indicate whether the following rate expression is acceptable: d[N2O5] = -k₁[N₂O₂] + K¸₁[NO₂][NO3] - K¸[NO₂]³ dtarrow_forwardIn a reaction of A + B to give C, another compound other than A, B or C may appear in the kinetic equation.arrow_forwardFor the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 →> NO₂+ NO3_(K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → → NO2 + O2 + NO (K2) NO + N2O5- NO2 + NO2 + NO2 (K3) d[N₂O5] __2k‚k₂[N2O5] Indicate whether the following rate expression is acceptable: dt k₁₁+ k₂arrow_forward
- Given the reaction R + Q → P, indicate the rate law with respect to R, with respect to P and with respect to P.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardk₁ Given the reaction A B, indicate k-1 d[A] (A). the rate law with respect to A: (B). the rate law with respect to B: d[B] dt dtarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT


