
Concept explainers
a)
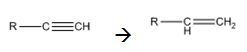
Interpretation:
How to convert an alkyne into an alkene, if necessary using more than one step, is to be shown.
Concept introduction:
To show:
How to convert an alkyne into an alkene, if necessary using more than one step.
Answer to Problem 36AP
Alkynes can be reduced to the corresponding alkenes in a single step as shown.
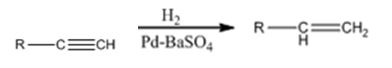
Explanation of Solution
Alkynes when treated with H2 in the presence of Pd-BaSO4 add two equivalents of hydrogen to yield an
Alkynes when treated with hydrogen in the presence of Pd-BaSO4 get reduced to alkenes in a single step.

b)
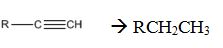
Interpretation:
How to convert an alkyne into an alkane, if necessary using more than one step, is to be shown.
Concept introduction:
Alkynes add two equivalents of hydrogen when treated with H2 in the presence of Raney nickel catalyst.
To show:
How to convert an alkyne into an alkane, if necessary using more than one step.
Answer to Problem 36AP
Alkynes can be reduced to the corresponding alkanes by using the step shown.
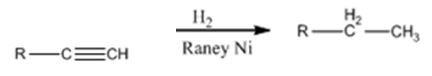
Explanation of Solution
Alkynes when treated with H2 in the presence of Raney Ni add two equivalents of hydrogen to yield the corresponding alkane.
Alkynes can be reduced to the corresponding alkanes by using the step shown.

c)
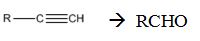
Interpretation:
How to convert an alkyne into an
Concept introduction:
Symmetrical internal alkynes yield an aldehyde as a single product during ozonolysis. The alkyne given can be converted in to a higher symmetrical alkyne by treating with NaNH2 in NH3 and RCl. The symmetrical alkyne thus obtained upon ozonolysis followed by reduction with Zn and acetic acid give an aldehyde as the only product.
To state:
How to convert an alkyne into an aldehyde , if necessary using more than one step.
Answer to Problem 36AP
The terminal alkyne can be converted into an aldehyde through the steps shown.
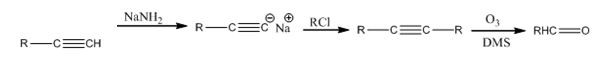
Explanation of Solution
The alkyne is first converted in to a higher alkyne by treating it with NaNH2 and then with RCl. The higher alkyne, being symmetrical, upon ozonolysis followed by reduction with Zn and acetic acid gets cleaved to give the aldehyde required as the product.
The terminal alkyne can be converted into an aldehyde through the steps shown.

d)
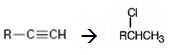
Interpretation:
How to convert an alkyne into secondary
Concept introduction:
Terminal alkynes when reduced with H2 in the presence of Pd-BaSO4 catalyst yield the corresponding alkenes. The alkenes when treated with HCl yield an alkyl halide following Markovnikov regiochemistry.
To state:
How to convert an alkyne into a secondary alkyl halide, if necessary using more than one step.
Answer to Problem 36AP
The alkyne can be converted into a secondary alkyl halide through the steps shown.
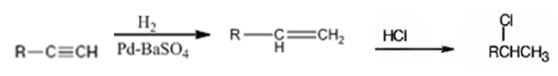
Explanation of Solution
The alkyne is first converted in to an alkene by treating it with H2 in the presence of Pd-BaSO4catalyst. Addition of HCl to alkenes follows Markovnikov regiochemistry. The chlorine atom adds to the carbon with one substituent and H adds to the terminal carbon without substituents to yield the product required.
An alkyne can be converted into a secondary alkyl halide through the steps shown.

e)
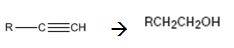
Interpretation:
How to convert (if necessary using more than one step) an alkyne into a primary alcohol that contains one more carbon is to be shown.
Concept introduction:
Alkynes can be converted into a primary alcohol through the following steps. The alkyne is first reduced to an alkene using H2 and Pd-BaSO4. Hydrohalogenation converts the alkene in to an alkyl halide which when treated with NaOH yields the required alcohol.
To state:
How to convert an alkyne into a primary alcohol, if necessary using more than one step.
Answer to Problem 36AP
The alkyne can be converted in to the alcohol through the steps shown.
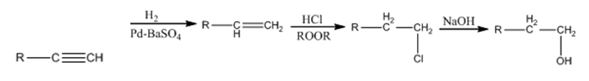
Explanation of Solution
The alkyne gets reduced to an alkene when treated with H2 and Pd-BaSO4. The alkene gets converted in to a primary alkyl halide when treated with HCl in the presence of a peroxide following anti Markovnikov regiochemistry. The alkyl halide yields the alcohol when the nucleophile OH- displaces Cl- when treated with NaOH.
The alkyne can be converted in to the alcohol through the steps shown.

f)
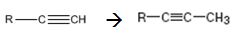
Interpretation:
How to convert an alkyne into its next higher alkyne, if necessary using more than one step, is to be shown.
Concept introduction:
Terminal alkynes are converted in to acetylides by treating with NaNH2 in liquid ammonia. The acetylides can be converted to higher alkynes by treating with alkyl halides having required number of carbon atoms.
To state:
How to convert an alkyne into its next higher alkyne, if necessary using more than one step.
Answer to Problem 36AP
The alkyne can be converted in to its next higher alkyne through the steps shown.
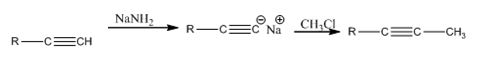
Explanation of Solution
The alkyne, being acidic, when treated with NaNH2 in liquid ammonia is converted in to the sodium acetylide. The acetylide upon treatment with methyl chloride yields the next higher alkyne.
The alkyne can be converted in to its next higher alkyne through the steps shown.

g)
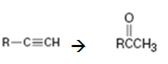
Interpretation:
How to convert an alkyne into a ketone, if necessary using more than one step, is to be shown.
Concept introduction:
Terminal alkynes when hydrated in the presence of dilute H2SO4 in the presence of HgSO4 are converted in to enols. The addition of water takes place following Markovnikov regiochemistry. The enols upon tautomerization yield
To state:
How to convert an alkyne into a ketone, if necessary, using more than one step.
Answer to Problem 36AP
The alkyne can be converted in to a ketone using the step shown.
Explanation of Solution
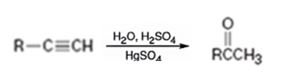
Addition of water takes place to the triple bond when the alkyne is treated with dilute H2SO4 in the presence of HgSO4 following Markovnikov regiochemistry. The OH adds on to the carbon with one substituent and H adds on to the terminal carbon with no substituent to yield an enol. The enol then undergoes tautomerization to give the ketone as the product.
The alkyne can be converted in to a ketone using the step shown.

h)
Interpretation:
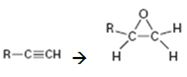
How to convert an alkyne into an epoxide, if necessary using more than one step, is to be shown.
Concept introduction:
Terminal alkynes are converted in to alkenes by treating with H2 in the presence of Pd-BaSO4. The alkenes are converted in to
To state:
How to convert an alkyne into an epoxide, if necessary using more than one step.
Answer to Problem 36AP
The alkyne can be converted in to an epoxide using the steps shown.
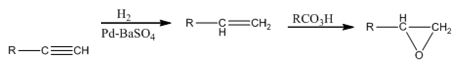
RCO3H=meta - chloroperoxybenzoic acid
Explanation of Solution
The terminal alkyne when reduced with H2 in the presence of Pd-BaSO4 yield the corresponding alkene. When the alkene is treated with meta -chloroperoxybenzoic acid, transfer of an oxygen atom from the acid to the double bond in alkene takes place with syn stereochemistry to yield the epoxide.
The alkyne can be converted in to the epoxide through the steps shown.

RCO3H=meta - chloroperoxybenzoic acid.
Want to see more full solutions like this?
Chapter 9 Solutions
Bundle: Organic Chemistry, 9th, Loose-Leaf + OWLv2, 4 terms (24 months) Printed Access Card
- You are a Quality Manager for a very well-known food ingredient company that produces umami powder, and you are responsible for setting specification limits. The net weight (in grams) of bags of unami powder is monitored by taking samples of six bags on an hourly basis during production. The label on every bag reports a contents of 1KG umami powder. The process mean is μ = 1012 g, and when the process is properly adjusted, it varies with σ = 11 g. QUESTION: Using all the available information, set the upper and lower specification limits.arrow_forward43) 10.00 ml of vinegar (active ingredient is acetic acid) is titrated to the endpoint using 19.32 ml of 0.250 M sodium hydroxide. What is the molarity of acetic acid in the vinegar? YOU MUST SHOW YOUR WORK. NOTE: MA x VA = MB x VBarrow_forward424 Repon Sheet Rates of Chemical Reactions : Rate and Order of 1,0, Deception B. Effect of Temperature BATH TEMPERATURE 35'c Yol of Oh نام Time 485 Buret rend ing(n) 12 194 16. 6 18 20 10 22 24 14 115 95 14738 2158235 8:26 CMS 40148 Total volume of 0, collected Barometric pressure 770-572 ml mm Hg Vapor pressure of water at bath temperature (see Appendix L) 42.2 Slope Compared with the rate found for solution 1, there is Using the ideal gas law, calculate the moles of O; collected (show calculations) times faster 10 Based on the moles of O, evolved, calculate the molar concentration of the original 3% 1,0, solution (sho calculations)arrow_forward
- Steps and explanations pleasearrow_forwardUse diagram to answer the following: 1.Is the overall rxn endo- or exothermic. Explain briefly your answer____________________2. How many steps in this mechanism?_____________3. Which is the rate determining step? Explain briefly your answer____________________4. Identify (circle and label) the reactants,the products and intermediate (Is a Cation, Anion, or a Radical?) Please explain and provide full understanding.arrow_forwardDraw the entire mechanism and add Curved Arrows to show clearly how electrons areredistributed in the process. Please explain and provide steps clearly.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

