
ORGANIC CHEMISTRY-PACKAGE >CUSTOM<
10th Edition
ISBN: 9781260028355
Author: Carey
Publisher: MCG CUSTOM
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 9.5, Problem 4P
Complete each of the following equations to show the conjugate acid and the conjugate base formed by proton transfer between the indicated species. Use curved arrows to show the flowof electrons, and specify whether the position of equilibrium lies to the side of reactants orproducts.
a) 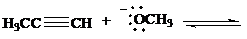
b) 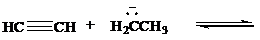
c) 
d) 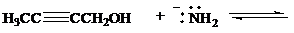
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Chapter 9 Solutions
ORGANIC CHEMISTRY-PACKAGE >CUSTOM<
Ch. 9.1 - Prob. 1PCh. 9.2 - Prob. 2PCh. 9.4 - How do bond distances and bond strengths change...Ch. 9.5 - Complete each of the following equations to show...Ch. 9.6 - Prob. 5PCh. 9.6 - Which of the alkynes of molecular formula C5H8 can...Ch. 9.7 - Give the structures of three isomeric dibromides...Ch. 9.7 - Prob. 8PCh. 9.9 - Write a series of equations showing how you could...Ch. 9.9 - Write a series of equations showing how to prepare...
Ch. 9.10 - Prob. 11PCh. 9.11 - Give the structure of the enol formed by hydration...Ch. 9.11 - Prob. 13PCh. 9.13 - Prob. 14PCh. 9.14 - Prob. 15PCh. 9 - Provide the IUPAC name for each of the following...Ch. 9 - Prob. 17PCh. 9 - All compounds in Problem 9.17 are isomers except...Ch. 9 - Prob. 19PCh. 9 - Write structural formulas for all the alkynes of...Ch. 9 - Prob. 21PCh. 9 - Prob. 22PCh. 9 - The alkane formed by hydrogenation of...Ch. 9 - Write the structure of the major organic product...Ch. 9 - Write the structure of the major organic product...Ch. 9 - When 2-heptyne was treated with aqueous sulfuric...Ch. 9 - Prob. 27PCh. 9 - Prob. 28PCh. 9 - Prob. 29PCh. 9 - Show by writing appropriate chemical equations how...Ch. 9 - Show by writing appropriate chemical equations how...Ch. 9 - Diphenylacetylene can be synthesized by the double...Ch. 9 - (Z)-9-tricosene [ (Z)-CH3(CH2)7CH=CH(CH2)12CH3 ]...Ch. 9 - Prob. 34PCh. 9 - Prob. 35PCh. 9 - Prob. 36PCh. 9 - Alkynes undergo hydroboration to give...Ch. 9 - Prob. 38DSPCh. 9 - Prob. 39DSPCh. 9 - Prob. 40DSPCh. 9 - Prob. 41DSPCh. 9 - Thinking Mechanistically About Alkynes The...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- When a proton becomes bonded to diethyl ether, by way of one of the unshared electron pairs on the oxygen atom, the result is In this structure the oxygen atom owns one electron from each of ____ shared pairs and two electrons from ____ unshared pair. The total number of electrons that belong to oxygen is ____. The formal charge on oxygen is ____. The correct Lewis structure for the conjugate acid of diethyl ether is ___________________________arrow_forwardThe following reactions illustrate Brnsted acid-base behavior. Complete each equation. a.HI(aq)+?H3O+(aq)+I(aq) b.NH3(l)+?NH4++NH2 c.H2C2O4(aq)+H2O(l)?+HC2O4(aq) d.H2N2O2(aq)+H2O(l)H3O+(aq)+? e.?+H2O(l)H3O+(aq)+CO32(aq)arrow_forwardIf the pH of a solution is 8.6, is the solution acidic or basic? How do you reach your conclusion? List in order the pH values of a solution that is neutral, one that is basic, and one that is acidic.arrow_forward
- The following are equivalent ways of asking about the acidity of an H atom: • What is the most acidic H on the molecule? • Which H is associated with the published pKa value? • Which H on the molecule is easiest to remove? • Which H on the molecule takes the least energy to remove? • Which bond to an H is most polarized? • For which H atom is removal least uphill in energy? • Which bond to an H atom, when broken, results in the lowest PE conjugate base? We will often find the last of these questions is easiest to answer. To do this, find all the different Hatoms on the molecule, and draw all possible conjugate bases.Only the lowest-energy one is the “real” conjugate base. Identify this structure, and you have found the most acidic H. Use this strategy to find the most acidic H on each of the following molecules. Note: Each structure hasat least three different kinds of H’s, so draw at least three unique conjugate bases for each.arrow_forwardList the following bases in order of their decreasing strength strongest base first: CN,H2O,HSO3,ClO,Cl.arrow_forwardA very strong base can remove a proton from methylamine:arrow_forward
- Complete the equation for the reaction between each Lewis acid-base pair. In each equation, label which starting material is the Lewis acid and which is the Lewis base; use curved arrows to show the flow of electrons in each reaction. In doing this problem, it is essential that you show valence electrons for all atoms participating in each reaction. (a) (b) (c) (d)arrow_forwardIn each of the following chemical equations, identify the conjugate acid—base pairs. a. HF(aq)+H2O(l)F-(aq)+H3O+(aq)b. CN(aq)+H2O(l)HCN(aq)+OH(aq) c. HCO3-(aq)+H2O(l)H2CO3(aq)+OH-(aq)arrow_forwardAnswer true or false to the following statements about the mechanism of acid-base reactions. (a) The acid and base must encounter each other by a collision in order for the proton to transfer. (b) All collisions between acids and bases result in proton transfer. (c) During an acid-base reaction the lone pair on the base fills the A-H antibonding sigma orbital.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY