
ORGANIC CHEMISTRY (LL)-W/SOLN.>CUSTOM<
10th Edition
ISBN: 9781259972348
Author: Carey
Publisher: MCG CUSTOM
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 9.5, Problem 4P
Complete each of the following equations to show the conjugate acid and the conjugate base formed by proton transfer between the indicated species. Use curved arrows to show the flowof electrons, and specify whether the position of equilibrium lies to the side of reactants orproducts.
a) 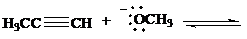
b) 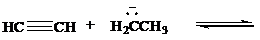
c) 
d) 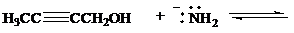
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Please correct answer and don't use hand rating
Q: Draw the molecular orbital energy level diagram for the following molecules.
1- The SF4 molecule is seesaw molecular geometry and has C2v point group.
2- The Mn(CO)s molecule with C4v point group is
square pyramidal.
Please correct answer and don't use hand rating
Chapter 9 Solutions
ORGANIC CHEMISTRY (LL)-W/SOLN.>CUSTOM<
Ch. 9.1 - Prob. 1PCh. 9.2 - Prob. 2PCh. 9.4 - How do bond distances and bond strengths change...Ch. 9.5 - Complete each of the following equations to show...Ch. 9.6 - Prob. 5PCh. 9.6 - Which of the alkynes of molecular formula C5H8 can...Ch. 9.7 - Give the structures of three isomeric dibromides...Ch. 9.7 - Prob. 8PCh. 9.9 - Write a series of equations showing how you could...Ch. 9.9 - Write a series of equations showing how to prepare...
Ch. 9.10 - Prob. 11PCh. 9.11 - Give the structure of the enol formed by hydration...Ch. 9.11 - Prob. 13PCh. 9.13 - Prob. 14PCh. 9.14 - Prob. 15PCh. 9 - Provide the IUPAC name for each of the following...Ch. 9 - Prob. 17PCh. 9 - All compounds in Problem 9.17 are isomers except...Ch. 9 - Prob. 19PCh. 9 - Write structural formulas for all the alkynes of...Ch. 9 - Prob. 21PCh. 9 - Prob. 22PCh. 9 - The alkane formed by hydrogenation of...Ch. 9 - Write the structure of the major organic product...Ch. 9 - Write the structure of the major organic product...Ch. 9 - When 2-heptyne was treated with aqueous sulfuric...Ch. 9 - Prob. 27PCh. 9 - Prob. 28PCh. 9 - Prob. 29PCh. 9 - Show by writing appropriate chemical equations how...Ch. 9 - Show by writing appropriate chemical equations how...Ch. 9 - Diphenylacetylene can be synthesized by the double...Ch. 9 - (Z)-9-tricosene [ (Z)-CH3(CH2)7CH=CH(CH2)12CH3 ]...Ch. 9 - Prob. 34PCh. 9 - Prob. 35PCh. 9 - Prob. 36PCh. 9 - Alkynes undergo hydroboration to give...Ch. 9 - Prob. 38DSPCh. 9 - Prob. 39DSPCh. 9 - Prob. 40DSPCh. 9 - Prob. 41DSPCh. 9 - Thinking Mechanistically About Alkynes The...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- when a 0.150 g sample of the compound was burned, it produced 0.138 g CO2 & 0.0566 g H2O. All the nitrogen in a different 0.200 g sample of the compound was converted to NH3, which was found to weigh 0.0238 g. Finally, the chlorine in a 0.125 g sample of the compound was converted to Cl- and by reacting it with AgNO3, all of the chlorine was recovered as the solid AgCl. The AgCl, when dried was found to weigh 0.251 g. What is the empirical formulaarrow_forwardPlease correct answer and don't use hand rating and don't use Ai solutionarrow_forwardHow to determine if this is N- ethylsaccharin or O-ethylsaccharin or a mixture of both based on chemical shifts.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY