
Write the structure of the major organic product isolated from the reaction of
Hydrogen (
Hydrogen (
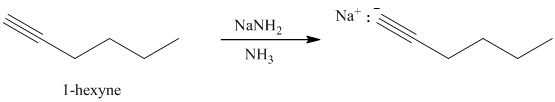
Sodium amide in liquid ammonia
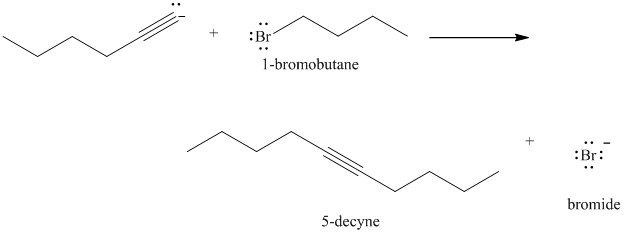
Product in part (c) treated with 1-bromobutane
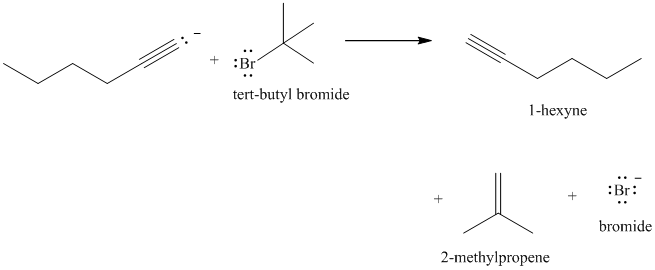
Product in part (c) treated with tert-butyl bromide
Hydrogen chloride (
Hydrogen chloride (
Chlorine (
Chlorine (
Aqueous sulfuric acid, mercury
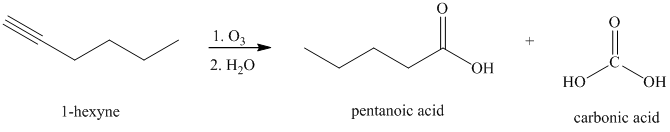
Ozone followed by hydrolysis
Interpretation:
The structure of the major product isolated from the reaction of
Concept introduction:
In the presence a metal catalyst such as platinum, palladium, nickel, or rhodium, two molar equivalents of hydrogen get added across the triple bond of an alkyne to yield an alkane. In hydrogenation reaction, two hydrogen atoms get added to each carbon atom across the triple bond.
Hydrogenation reaction of alkynes to alkenes using the Lindlar catalyst is stereoselective. In this reaction only the cis alkene is produced.
Terminal alkynes react with
Addition of hydrogen halides to alkynes gives alkenyl halides. The regioselectivity of this reaction follows Markovnikov’s rule. The hydrogen atom from hydrogen halide adds to the carbon in the alkyne that has the more number of hydrogens, and the halide adds to the carbon with the lower number of hydrogens.
In presence of excess of hydrogen halide, the sequential addition of two molecules of hydrogen halide to the carbon-carbon triple bond yields geminal dihalides.
The reaction of chlorine and bromine with alkynes yields tetrahaloderivatives. In this reaction, two molecules of the halogen get added to the triple bond. During the reaction, a dihaloalkene is get formed. When alkyne and the halogen are present in equimolar quantity, the dihaloalkene intermediate get formed and isolated. The stereochemistry of addition reaction is anti.
Alkynes, when subjected to ozonolysis followed by hydrolysis, produce carboxylic acids.
Answer to Problem 24P
Solution:
a)
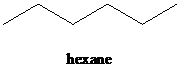
b)
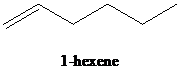
c)
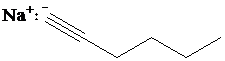
d)
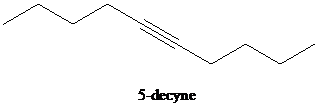
e)
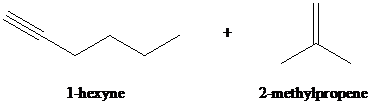
f)
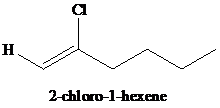
g)
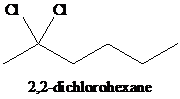
h)

i)
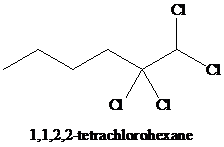
j)
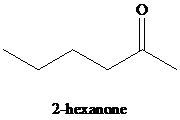
k)
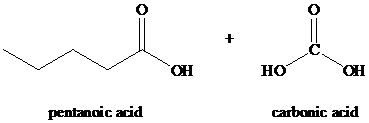
Explanation of Solution
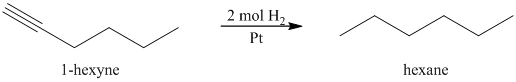
a) The reaction of
In the presence a metal catalyst such as platinum, palladium, nickel, or rhodium, two molar equivalents of hydrogen get added across the triple bond of an alkyne to yield an alkane. In case of
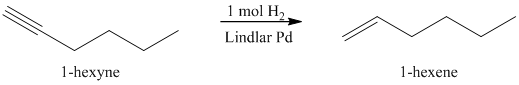
b) The reaction of
Reduction over Lindlar palladium catalyst with one mole of hydrogen partially reduces an alkyne to the corresponding cis alkene.
c) The reaction of
Terminal alkynes react with
d) The reaction of
In the reaction of alkyl halide and sodium acetylide, the acetylide ion acts as a nucleophile. It displaces halide from carbon and forming a new carbon-carbon bond. This reaction follows
The product formed in part (c) is the anion formed by
The product formed is
e) The reaction of
Acetylide anions are much more basic than hydroxide. Acetylide anions react with secondary and tertiary alkyl halides by elimination. Tert-butyl bromide is a tertiary alkyl halide and does not react by the
The product formed in part (c) is the anion formed by
f) The reaction of
Hydrogen halides add to alkynes to form alkenyl halides. When
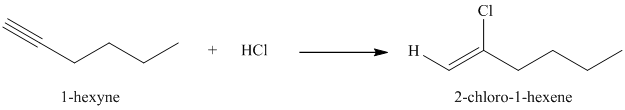
g) The reaction of
In presence of excess of hydrogen halide, the sequential addition of two molecules of hydrogen halide to the carbon-carbon triple bond yields geminal dihalides. The second mole addition of hydrogen halide to the initially formed alkenyl halide is done in accordance with Markovnikov’s rule. Overall, both protons get attached to the same carbon and both halogens to the adjacent carbon atom. In
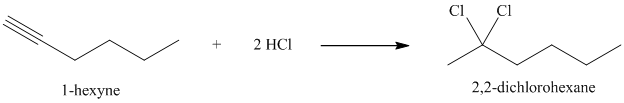
h) The reaction of
When one mole of halogen molecule is added to an alkyne, the product formed is a dihaloalkene. One halogen atom gets attached to each of the triple bonded carbon atoms, and it is reduced to a double bond.
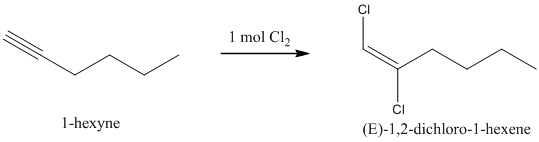
i) The reaction of
Alkynes react with two moles of chlorine to yield tetrachloroalkanes. Two molecules of the chlorine add to the triple bond. The stereochemistry of addition is anti.
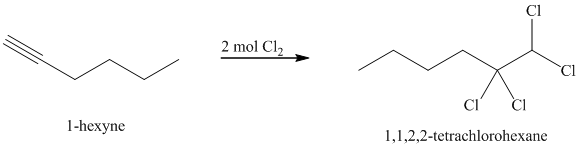
j) The reaction of
Hydration of alkynes employs aqueous sulfuric acid as the reaction medium and mercury(II) sulfate as a catalyst. The product formed is an enol which tautomerizes to the corresponding ketone. Markovnikov’s rule is followed in hydration of alkynes. Terminal alkynes yield methyl substituted ketones.
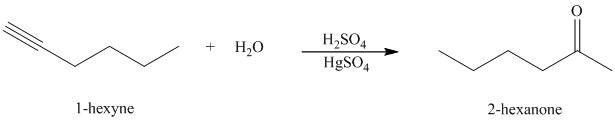
k) The reaction of
Alkynes, when subjected to ozonolysis followed by hydrolysis, produce carboxylic acids. If carbonic acid is one of the products, it dissociates into carbon dioxide and water.
Want to see more full solutions like this?
Chapter 9 Solutions
ORGANIC CHEMISTRY (LL)-W/SOLN.>CUSTOM<
- Hand written equations pleasearrow_forward> each pair of substrates below, choose the one that will react faster in a substitution reaction, assuming that: 1. the rate of substitution doesn't depend on nucleophile concentration and 2. the products are a roughly 50/50 mixture of enantiomers. Substrate A Substrate B Faster Rate X Ś CI (Choose one) (Choose one) CI Br Explanation Check Br (Choose one) © 2025 McGraw Hill LLC. All Rights Farrow_forwardNMR spectrum of ethyl acetate has signals whose chemical shifts are indicated below. Which hydrogen or set of hydrogens corresponds to the signal at 4.1 ppm? Select the single best answer. The H O HỌC—C—0—CH, CH, 2 A ethyl acetate H NMR: 1.3 ppm, 2.0 ppm, 4.1 ppm Check OA B OC ch B C Save For Later Submit Ass © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center |arrow_forward
- How many signals do you expect in the H NMR spectrum for this molecule? Br Br Write the answer below. Also, in each of the drawing areas below is a copy of the molecule, with Hs shown. In each copy, one of the H atoms is colored red. Highlight in red all other H atoms that would contribute to the same signal as the H already highlighted red Note for advanced students: In this question, any multiplet is counted as one signal. 1 Number of signals in the 'H NMR spectrum. For the molecule in the top drawing area, highlight in red any other H atoms that will contribute to the same signal as the H atom already highlighted red. If no other H atoms will contribute, check the box at right. Check For the molecule in the bottom drawing area, highlight in red any other H atoms that will contribute to the same signal as the H atom already highlighted red. If no other H atoms will contribute, check the box at right. O ✓ No additional Hs to color in top molecule ง No additional Hs to color in bottom…arrow_forwardin the kinetics experiment, what were the values calculated? Select all that apply.a) equilibrium constantb) pHc) order of reactiond) rate contstantarrow_forwardtrue or false, given that a 20.00 mL sample of NaOH took 24.15 mL of 0.141 M HCI to reach the endpoint in a titration, the concentration of the NaOH is 1.17 M.arrow_forward
- in the bromothymol blue experiment, pKa was measured. A closely related compound has a Ka of 2.10 x 10-5. What is the pKa?a) 7.1b) 4.7c) 2.0arrow_forwardcalculate the equilibrium concentration of H2 given that K= 0.017 at a constant temperature for this reaction. The inital concentration of HBr is 0.050 M.2HBr(g) ↔ H2(g) + Br2(g)a) 4.48 x 10-2 M b) 5.17 x 10-3 Mc) 1.03 x 10-2 Md) 1.70 x 10-2 Marrow_forwardtrue or falsegiven these two equilibria with their equilibrium constants:H2(g) + CI2(l) ↔ 2HCI(g) K= 0.006 CI2(l) ↔ CI2(g) K= 0.30The equilibrium contstant for the following reaction is 1.8H2(g) + CI2 ↔ 2HCI(g)arrow_forward
- I2(g) + CI2(g) ↔ 2ICIK for this reaction is 81.9. Find the equilibrium concentration of I2 if the inital concentration of I2 and CI2 are 0.010 Marrow_forwardtrue or false,the equilibrium constant for this reaction is 0.50.PCI5(g) ↔ PCI3(g) + CI2(g)Based on the above, the equilibrium constant for the following reaction is 0.25.2PCI5(g) ↔. 2PCI3(g) + 2CI2(g)arrow_forwardtrue or false, using the following equilibrium, if carbon dioxide is added the equilibrium will shift toward the productsC(s) + CO2(g) ↔ 2CO(g)arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

