
Concept explainers
Interpretation:
The series of equations for the preparation of each of the given compound from the designated starting material using suitable organic or inorganic reagents is to be written.
Concept introduction:
The conjugate base of alkyne acts as a good nucleophile. On reaction with primary
In hydration of alkynes, a molecule of water gets added to a molecule of alkyne.Hydrogen atom gets attached to the less substituted triple bonded carbon atom, and hydroxyl groupgetsbonded to the more substituted triple bonded carbon atom and forms a
Geminal and vicinal dihalides, on reaction with a strong base, undergo double dehydrohalogenation and form alkynes.
On halogenation, alkynes form vicinal dihalides and, on reaction with hydrogen halides, they form geminal dihalides in two steps.
Primary alcohol can be converted to primary alkyl bromide by reaction with sodium bromide in acidic condition.
Answer to Problem 36P
Solution:
a) 
b) 
c) 
d) 
e) 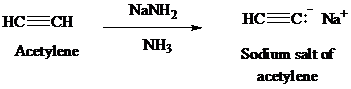

f) 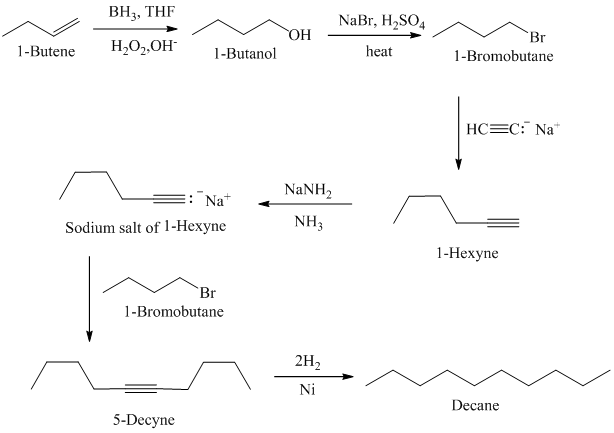
g) 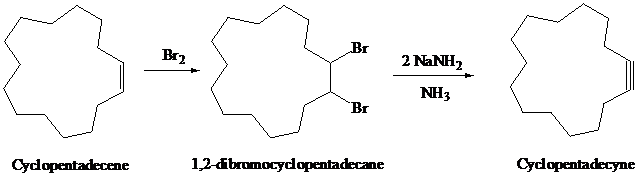
h) 
Explanation of Solution
a)
The
The synthesis of

b)
The
The synthesis of

c)
The
The synthesis of

d)
Acetylene on treatment with sodium amide, gives sodium salt of acetylene. The sodium salt of acetylene on treatment with ethyl bromide gives
The synthesis of

e)
Acetylene on treatment with sodium amide, gives sodium salt of acetylene. In presence of organoboron,
The synthesis of
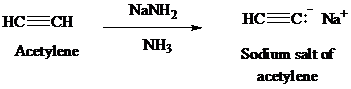

f)
In presence of organoboron,
The synthesis of

g)
Cyclopentadecene on halogenation with
The synthesis of
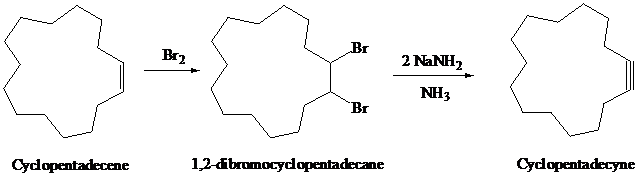
h) 
In presence of base sodium amide, the given alkyne gives sodium salt of alkyne. The sodium salt of alkyne on treatment with bromomethane adds one methyl group to alkyne, which on partial hydrogenation with Lindlar catalyst gives required cis alkene.
The synthetic route is as follows:

Want to see more full solutions like this?
Chapter 9 Solutions
ORGANIC CHEMISTRY (LL)-W/SOLN.>CUSTOM<
- What is the product of the following reaction? Please include a detailed explanation of what is happening in this question. Include a drawing showing how the reagent is reacting with the catalyst to produce the correct product. The correct answer is IV.arrow_forwardPlease complete the reactions, thank youarrow_forwardConsider the synthesis. What is compound Y? Please explain what is happening in this question. Provide a detailed explanation and a drawing to show how the compound Y creates the product. The correct answer is D.arrow_forward
- What would be the major product of the following reaction? Please include a detailed explanation of what is happening in this question. Include steps and a drawing to show this reaction proceeds and how the final product is formed. The correct answer is B. I put answer D and I don't really understand what is going on in the question.arrow_forwardWhat is the product of the following reaction? Please explain what is happening in this question. Provide a detailed explanation and a drawing showing how the reagent is reacting with the catalysts to product the correct product. The correct answer is B.arrow_forwardWhat is the missing intermediate 1 and the final product 2. Please include a detailed explanation explaining the steps of malonic ester synthesis. Please include drawings of the intermediate and how it occurs and how the final product is former.arrow_forward
- What would be the reagents and conditions above and below the arrow that will complete the proposed acetoacetic ester synthesis? If it cannot be done efficiently, then I will choose that answer. There could be 2 or 4 reagents involved. Please provide a detailed explanation and drawings showing how it would proceed with the correct reagents.arrow_forwardFor benzene, the ∆H° of vaporization is 30.72 kJ/mol and the ∆S° of vaporization is 86.97 J/mol・K. At 1.00 atm and 228.0 K, what is the ∆G° of vaporization for benzene, in kJ/mol?arrow_forwardThe reaction Q(g) + R(g) → Z(l) is shown to be exothermic. Which of the following is true concerning the reaction. it is spontaneous only at High T, it is spontaneous at low T it is nonspontaneous at all T it is spontanrous at all T. it is non spontaneous only at low T.arrow_forward
- The reaction Q(g) + R(g) → Z(l) is shown to be exothermic. Which of the following is true concerning the reactionarrow_forwardWhich of the following has the largest standard molar entropy, S° (298.15 K) He H2 NaCl KBr Hgarrow_forwardWhich of the following is true for a particular reaction if ∆G° is -40.0 kJ/mol at 290 K and –20.0 kJ/mol at 390 K?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
