
(a)
Interpretation:
A multi-step synthesis has to be designed for the given conversion.
Concept Introduction:
Target molecule is nothing but the desired product.
Adding
The elimination reaction should be carried out under SN2 condition i.e. with strong nucleophile. So there will be no compensating substitution reaction.
The bulky base is used in the elimination reaction to maximize the amount of elimination product.
To prepare cyclic ether, the
Addition of water to the given starting material creates bifunctional compound.
(b)
Interpretation:
A multi-step synthesis has to be designed for the given conversion.
Concept Introduction:
Target molecule is nothing but the desired product.
Adding
The elimination reaction should be carried out under SN2 condition i.e. with strong nucleophile. So there will be no compensating substitution reaction.
The bulky base is used in the elimination reaction to maximize the amount of elimination product.
To prepare cyclic ether, the alkyl halide and alcohol must be a part of the same molecule.
Addition of water to the given starting material creates bifunctional compound.
(c)
Interpretation:
A multi-step synthesis has to be designed for the given conversion.
Concept Introduction:
Target molecule is nothing but the desired product.
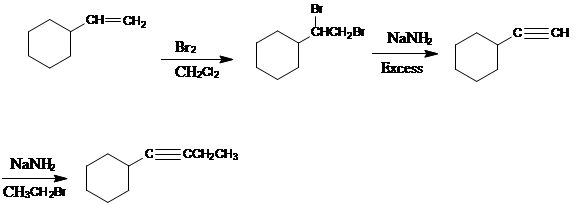 Adding
Adding
The elimination reaction should be carried out under SN2 condition i.e. with strong nucleophile. So there will be no compensating substitution reaction.
The bulky base is used in the elimination reaction to maximize the amount of elimination product.
To prepare cyclic ether, the alkyl halide and alcohol must be a part of the same molecule.
Addition of water to the given starting material creates bifunctional compound.
(d)
Interpretation:
A multi-step synthesis has to be designed for the given conversion.
Concept Introduction:
Target molecule is nothing but the desired product.
Adding
The elimination reaction should be carried out under SN2 condition i.e. with strong nucleophile. So there will be no compensating substitution reaction.
The bulky base is used in the elimination reaction to maximize the amount of elimination product.
To prepare cyclic ether, the alkyl halide and alcohol must be a part of the same molecule.
Addition of water to the given starting material creates bifunctional compound.
Want to see the full answer?
Check out a sample textbook solution
Chapter 9 Solutions
Organic Chemistry; Organic Chemistry Study Guide A Format: Kit/package/shrinkwrap
- can you please draw out and list step-by-step the synthetic strategy for this rxn? thank you sm in advancearrow_forwardSteps and explanations pleasearrow_forwardUse diagram to answer the following: 1.Is the overall rxn endo- or exothermic. Explain briefly your answer____________________2. How many steps in this mechanism?_____________3. Which is the rate determining step? Explain briefly your answer____________________4. Identify (circle and label) the reactants,the products and intermediate (Is a Cation, Anion, or a Radical?) Please explain and provide full understanding.arrow_forward
- Draw the entire mechanism and add Curved Arrows to show clearly how electrons areredistributed in the process. Please explain and provide steps clearly.arrow_forward15) Create Lewis structure Br Brarrow_forwardLIOT S How would you make 200. mL of a 0.5 M solution of CuSO4 5H2O from solid copper (II) sulfate? View Rubricarrow_forward
- Steps and explantions pleasearrow_forwardMatch the denticity to the ligand. Water monodentate ✓ C₂O2 bidentate H₂NCH₂NHCH2NH2 bidentate x EDTA hexadentate Question 12 Partially correct Mark 2 out of 2 Flag question Provide the required information for the coordination compound shown below: Na NC-Ag-CN] Number of ligands: 20 Coordination number: 2✔ Geometry: linear Oxidation state of transition metal ion: +3 x in 12 correct out of 2 question Provide the required information for the coordination compound shown below. Na NC-Ag-CN] Number of ligands: 20 Coordination number: 2 Geometry: linear 0 Oxidation state of transition metal ion: +3Xarrow_forwardCan you explain step by step behind what the synthetic strategy would be?arrow_forward
- Please explain step by step in detail the reasoning behind this problem/approach/and answer. thank you!arrow_forward2. Predict the product(s) that forms and explain why it forms. Assume that any necessary catalytic acid is present. .OH HO H₂N OHarrow_forwardconsider the rate of the reaction below to be r. Whats the rate after each reaction? Br + NaCN CN + NaBr a. Double the concentration of alkyl bromide b. Halve the concentration of the electrophile & triple concentration of cyanide c. Halve the concentration of alkyl chloridearrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
