
The exergy destruction associated with each process of the Brayton cycle and the exergy of the exhaust gases at the exit of the regenerator.
Answer to Problem 150P
The exergy destruction associated with process 1-2 for Brayton cycle is
The exergy destruction associated with process 3-4 for Brayton cycle is
The exergy destruction associated with regeneration process for Brayton cycle is
The exergy destruction associated with process 5-3 for Brayton cycle is
The exergy destruction associated with process 6-1 for Brayton cycle is
The exergy of the exhaust gases at the exit of the regenerator is
Explanation of Solution
Draw
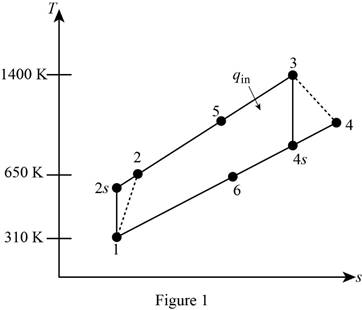
Write the expression of pressure ratio for the regenerative Brayton cycle
Here, pressure at state 2 is
Write the pressure ratio and pressure relation for the process 3-4.
Here, pressure at state 3 is
Write the expression of efficiency of the turbine
Here, enthalpy at state 3 is
Write the expression of heat added due to regeneration
Here, the effectiveness of the regenerator is
Write the expression of net work output of the regenerative Brayton cycle
Here, the work output by the turbine is
Write the expression of heat input to the regenerative Brayton cycle
Write the expression of heat rejected by the regenerative Brayton cycle
Write the expression of specific enthalpy at state 6
Write the specific enthalpy relation for the regenerator.
Write the expression of exergy destruction associated with the process 1-2 for Brayton cycle
Here, the gas constant of air is R, entropy of air at state 2 as a function of temperature only is
Write the expression of exergy destruction for process 3-4
Here, entropy of air at state 3 as a function of temperature is
Write the expression of exergy destruction for Brayton cycle
Here, entropy of air at state 5 as a function of temperature alone is
Write the expression of exergy destruction for process 5-3
Here, the temperature of the heat source is
Write the expression of exergy destruction for process 6-1
Here, the temperature of the sink is
Write the expression of stream exergy at the exit of the regenerator (state 6)
Here, the specific enthalpy of the surroundings is
Write the expression of change entropy for the exit of the regenerator
Here, entropy of air at the surroundings as a function of temperature alone is
Conclusion:
Refer Table A-17, “Ideal gas properties of air”, obtain the properties of air at 310 K
Substitute 900 kPa for
Substitute
Refer Table A-17, “Ideal gas properties of air”, obtain the properties of air at 50.06
Substitute
Substitute 0.80 for
Substitute
Substitute
Substitute
Substitute 310.24
Substitute 659.84
Refer Table A-17, “Ideal gas properties of air”, obtain the properties of air at 310 K
Substitute 300 K for
Thus, the exergy destruction associated with process 1-2 for Brayton cycle is
Substitute 300 K for
Thus, the exergy destruction associated with process 3-4 for Brayton cycle is
Substitute 300 K for
Thus, the exergy destruction associated with regeneration process for Brayton cycle is
Substitute 300 K for
Thus, the exergy destruction associated with process 5-3 for Brayton cycle is
Substitute 300 K for
Thus, the exergy destruction associated with process 6-1 for Brayton cycle is
Refer Table A-17, “Ideal gas properties of air”, obtain the properties of air at 300 K
Substitute
Substitute
Thus, the exergy of the exhaust gases at the exit of the regenerator is
Want to see more full solutions like this?
Chapter 9 Solutions
CONNECT FOR THERMODYNAMICS: AN ENGINEERI
- According to the principles and steps above, draw the kinematic diagram of following mechanisms. Mark the appropriate scale, calculates the degree of freedom. NO.1 NO.2 NO: 3 NO.: 4arrow_forwardAn office building is planned with a lateral-force-resisting system designed for earthquake resistance in aseismic zone. The seismic capacity of the proposed system, expressed as a force factor, is assumed tofollow a lognormal distribution with a median of 6.5 and a standard deviation of 1.5. The ground motionfrom the largest expected earthquake at the site is estimated to correspond to an equivalent force factor of 5.5.(a) What is the estimated probability that the building will experience damage when subjected to the largest expected earthquake? (b) If the building survives (i.e., experiences no damage) during a previous moderate earthquake with aforce factor of 4.0, what is the updated probability of failure of the building under the largest expectedearthquake?(c) Suppose future occurrences of the largest expected earthquake follow a Poisson process with a mean return period of 500 years. Assuming that damage events from different earthquakes are statisticallyindependent,…arrow_forwardDuring a plant visit, it was noticed that a 12-m-long section of a 10-cm-diameter steam pipe is completely exposed to the ambient air. The temperature measurements indicate that the average temperature of the outer surface of the steam pipe is 75°C when the ambient temperature is 5°C. There are also light winds in the area at 10 km/h. The emissivity of the outer surface of the pipe is 0.8, and the average temperature of the surfaces surrounding the pipe, including the sky, is estimated to be 0°C. Determine the amount of heat lost from the steam during a 10-h-long work day. Steam is supplied by a gas-fired steam generator that has an efficiency of 80 percent, and the plant pays $1.05/therm of natural gas. If the pipe is insulated and 90 percent of the heat loss is saved, determine the amount of money this facility will save a year as a result of insulating the steam pipes. Assume the plant operates every day of the year for 10 h. State your assumptions.arrow_forward
- An old fashioned ice cream kit consists of two concentric cylinders of radii Ra and Rb. The inner cylinder is filled with milk and ice cream ingredients while the space between the two cylinders is filled with an ice-brine mixture. Ice cream begins to form on the inner surface of the inner cylinder. To expedite the process, would you recommend rotating the inner cylinder? Justify your recommendation. icecream/ ice-brine Ra Rbarrow_forwardFind temperatures STRICTLY USING RITZ APPROXIMATION METHODarrow_forwardSolve this Problem using RITZ APPROXIMATION. STEP BY STEParrow_forward
- B/40 The body is constructed of a uniform square plate, a uniform straight rod, a uniform quarter‐circular rod, and a particle (negligible dimensions). If each part has the indicated mass, determine the mass moments of inertia of the body about the x‐, y‐, and z‐axes. Answer Given.arrow_forward(read image) Answer:arrow_forward(read image) Answer Givenarrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





