
Concept explainers
(a)
Interpretation:
The reason behind less volatility of hexane than diethyl ether has to be described.
(a)
Explanation of Solution
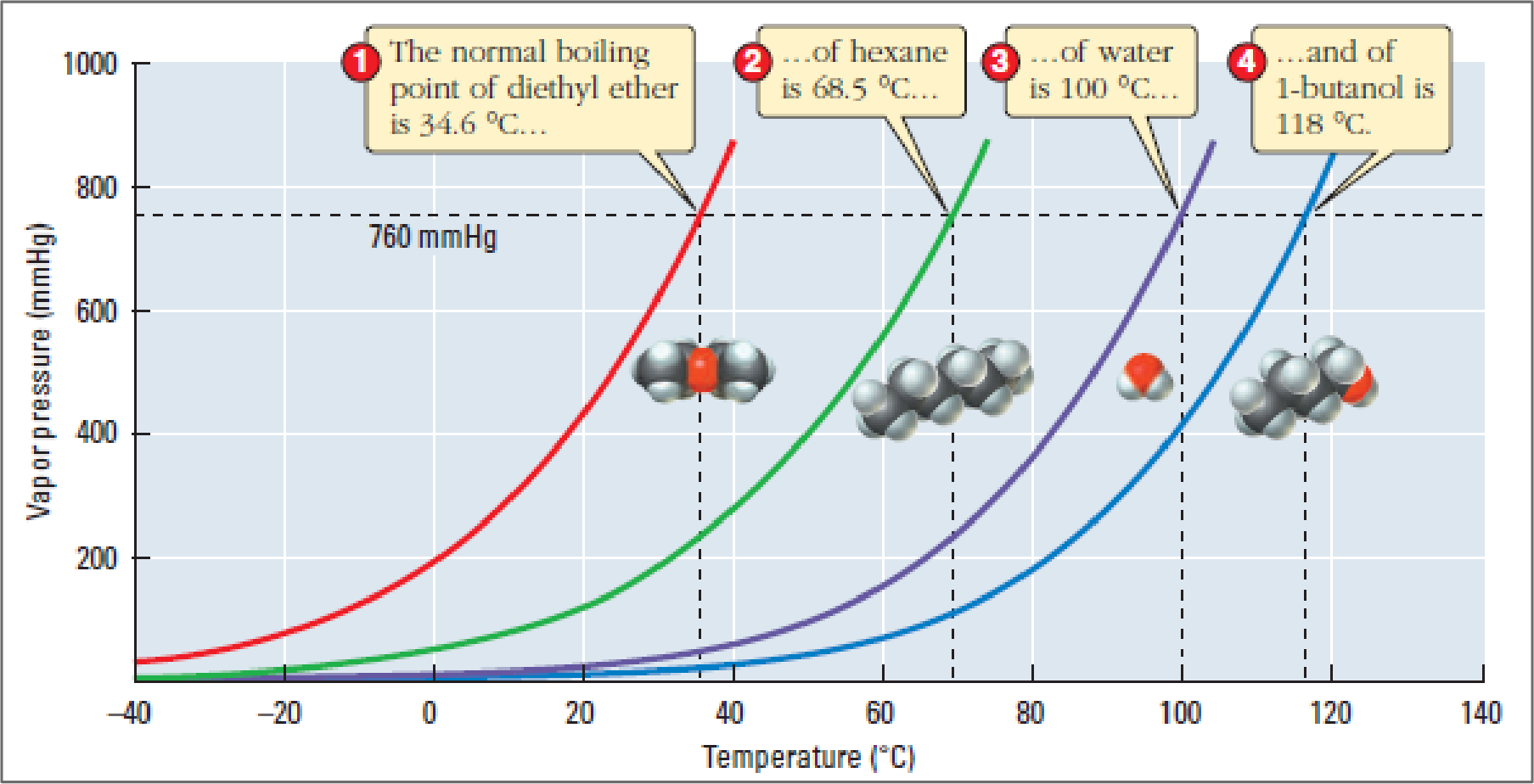
Figure 1
Vapour pressure of a liquid is defined as the pressure of the vapour when liquid and vapour are in dynamic equilibrium. It increases with increase in temperature and a liquid with stronger intermolecular force of attractions has a lower vapour pressure at a given temperature.
If a liquid is less volatile, then it must have a lower vapour pressure which can be accounted for its stronger intermolecular force of attraction. Examining the above figure, it can be concluded that hexane has a lower vapour pressure than diethyl ether at any temperature. The non-covalent intermolecular forces of attraction acting between the molecules of hexane is London forces while in diethyl ether they are dipole-dipole forces and London forces. Because liquid hexane has a lower vapour pressure, it can be predicted that the larger hexane molecules experience larger collective intermolecular London forces than diethyl ether.
So due to this reason, hexane is less volatile than diethyl ether.
(b)
Interpretation:
The temperature at which 1- butanol have a pressure of
(b)
Answer to Problem ISP
The temperature of 1-butanol at
Explanation of Solution
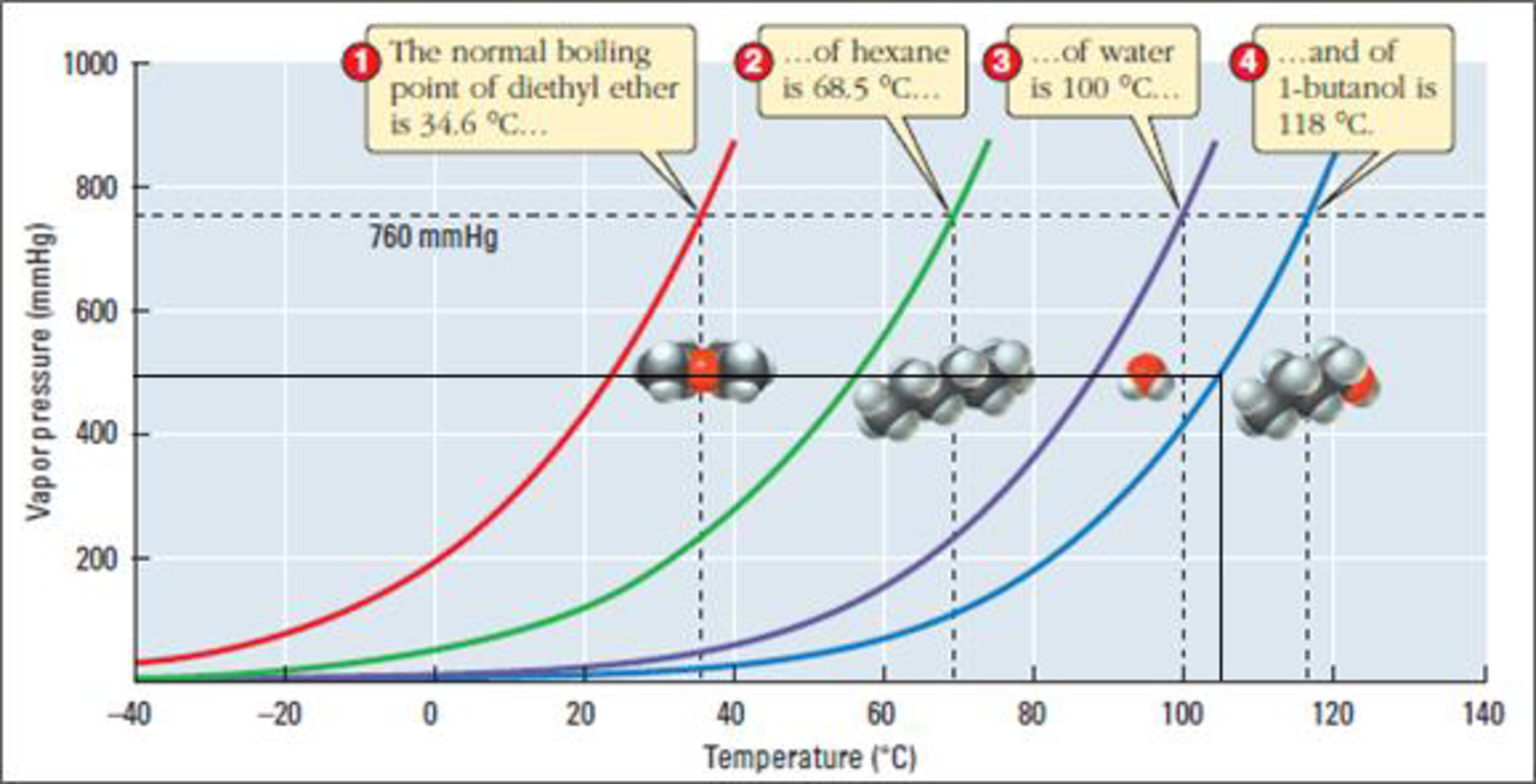
Figure 2
Examine the above figure carefully. Draw a horizontal line from
(c)
Interpretation:
The reason why the boiling point of 1-butanol is greater than that of water has to be described.
(c)
Explanation of Solution
The liquid having greater boiling point means it has stronger collective intermolecular forces. Both water and 1-butanol can experience hydrogen bonding interactions. Water is a small molecule with a total of
(c)
Interpretation:
The substance which would evaporate immediately and which would remain as liquid has to be determined.
(c)
Explanation of Solution
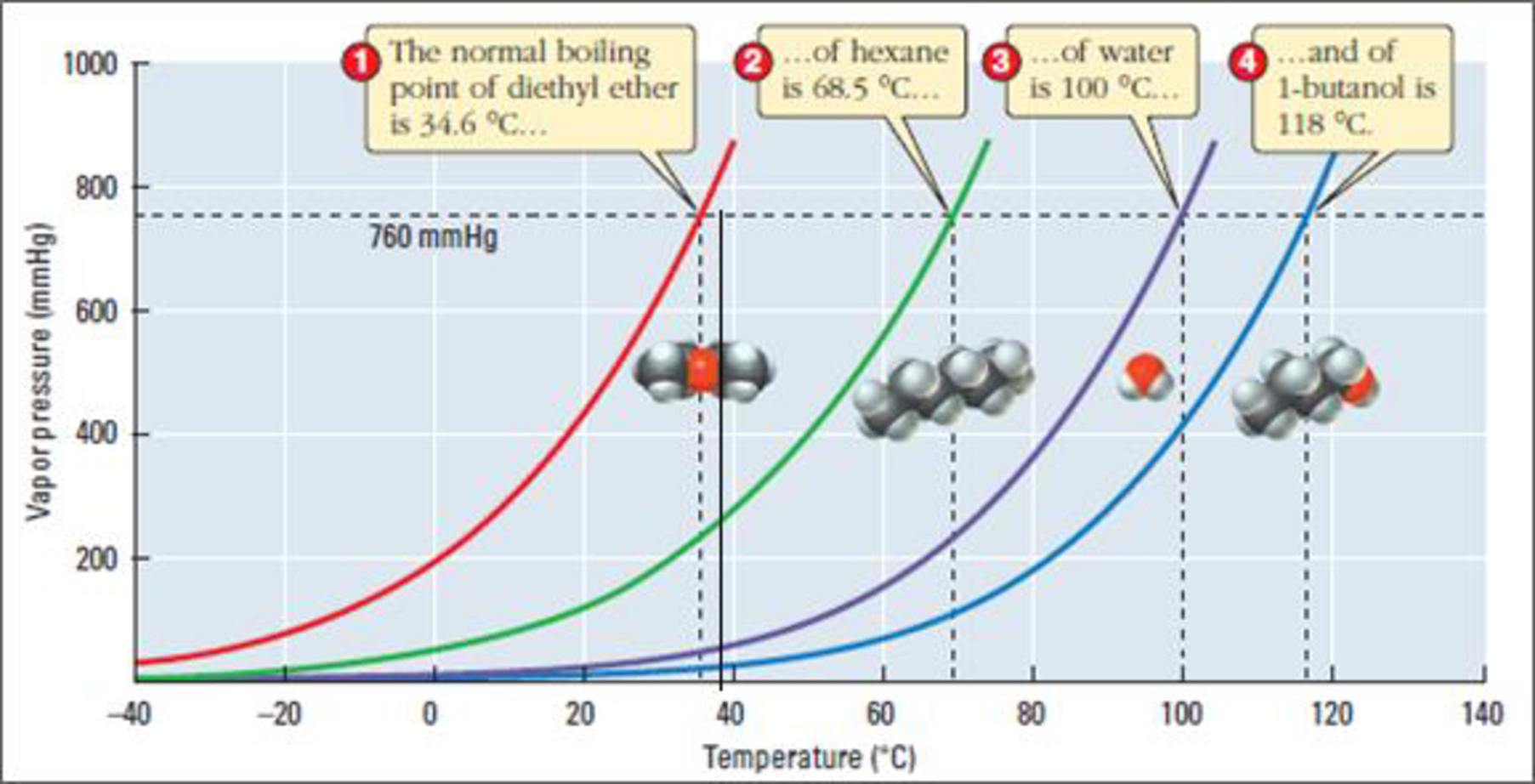
Figure 3
The boiling point of diethyl ether is
(d)
Interpretation:
The
Concept Introduction:
Clausius-Clapeyron equation:
Where,
P1 and P2 are two sets of pressures and T1 and T2 are two sets of absolute temperatures.
R is universal gas constant.
(d)
Answer to Problem ISP
The
Explanation of Solution
Given data:
The normal boiling point of 1-butanol is
Clausius-Clapeyron equation:
Substituting all the data in the above equation and solving for
Therefore, the
Want to see more full solutions like this?
Chapter 9 Solutions
OWLV2 FOR MOORE/STANITSKI'S CHEMISTRY:
- true or false, given that a 20.00 mL sample of NaOH took 24.15 mL of 0.141 M HCI to reach the endpoint in a titration, the concentration of the NaOH is 1.17 M.arrow_forwardin the bromothymol blue experiment, pKa was measured. A closely related compound has a Ka of 2.10 x 10-5. What is the pKa?a) 7.1b) 4.7c) 2.0arrow_forwardcalculate the equilibrium concentration of H2 given that K= 0.017 at a constant temperature for this reaction. The inital concentration of HBr is 0.050 M.2HBr(g) ↔ H2(g) + Br2(g)a) 4.48 x 10-2 M b) 5.17 x 10-3 Mc) 1.03 x 10-2 Md) 1.70 x 10-2 Marrow_forward
- true or falsegiven these two equilibria with their equilibrium constants:H2(g) + CI2(l) ↔ 2HCI(g) K= 0.006 CI2(l) ↔ CI2(g) K= 0.30The equilibrium contstant for the following reaction is 1.8H2(g) + CI2 ↔ 2HCI(g)arrow_forwardI2(g) + CI2(g) ↔ 2ICIK for this reaction is 81.9. Find the equilibrium concentration of I2 if the inital concentration of I2 and CI2 are 0.010 Marrow_forwardtrue or false,the equilibrium constant for this reaction is 0.50.PCI5(g) ↔ PCI3(g) + CI2(g)Based on the above, the equilibrium constant for the following reaction is 0.25.2PCI5(g) ↔. 2PCI3(g) + 2CI2(g)arrow_forward
- true or false, using the following equilibrium, if carbon dioxide is added the equilibrium will shift toward the productsC(s) + CO2(g) ↔ 2CO(g)arrow_forward2S2O2/3- (aq) + I2 (aq) ---> S4O2/6- (aq) +2I- (aq) Experiment I2 (M) S2O3- (M) Initital Rate (M/s) 1 0.01 0.01 0.0004 2 0.01 0.02 0.0004 3 0.02 0.01 0.0008 Calculate the overall order for this reaction using the table data a) 3b) 0c) 2d) 1arrow_forwardthe decomposition of N2O5 is the first order with a half-life of 1.98 minutes. If the inital concentration of N2O5 is 0.200 M, what is the concentration after 6 minutes?a) 0.612 Mb) 0.035 Mc) 0.024 Md) 0.100 Marrow_forward
- 20.00 mL of 0.150 M HCI is titrated with 0.075 M NaOH. What volume of NaOH is needed?a) 50 mLb) 20 mLc) 40 mLd) 26.66 mLarrow_forward20.00 mL of 0.150 M NaOH is titrated with 37.75 mL of HCI. What is the molarity of the HCI?a) 0.150 Mb) 0.079 Mc) 0.025 Md) 0.050 Marrow_forwardin the following reaction, the OH- acts as which of these?NO2- (aq) + H2O (l) ⇌ OH- (aq) + HNO2 (aq)a) not a weak acidb) basec) acidarrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





