
EBK GENERAL, ORGANIC, AND BIOLOGICAL CH
7th Edition
ISBN: 8220100853180
Author: STOKER
Publisher: CENGAGE L
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 9, Problem 9.81EP
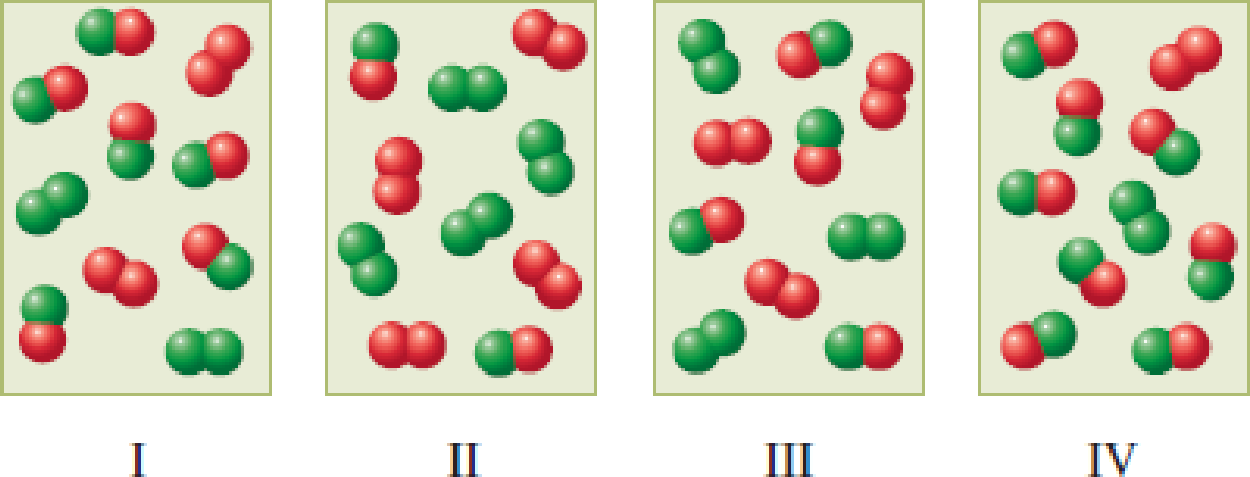
The following four diagrams represent gaseous equilibrium mixtures for the reaction A2 + B2 → 2AB at four different temperatures. For which of the diagrams is the numerical value of the equilibrium constant the largest? (A atoms are red and B atoms are green in the various diagrams.)
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
For the compound: C8H17NO2
Use the following information to come up with a plausible structure:
8
This compound has "carboxylic acid amide" and ether functional groups.
The peaks at 1.2ppm are two signals that are overlapping one another.
One of the two signals is a doublet that represents 6 hydrogens; the
other signal is a quartet that represents 3 hydrogens.
Vnk the elements or compounds in the table below in decreasing order of their boiling points. That is, choose 1 next to the substance with the highest bolling
point, choose 2 next to the substance with the next highest boiling point, and so on.
substance
C
D
chemical symbol,
chemical formula
or Lewis structure.
CH,-N-CH,
CH,
H
H 10: H
C-C-H
H H H
Cale
H 10:
H-C-C-N-CH,
Bri
CH,
boiling point
(C)
Сен
(C) B
(Choose
Please help me find the 1/Time, Log [I^-] Log [S2O8^2-], Log(time) on the data table. With calculation steps. And the average for runs 1a-1b. Please help me thanks in advance. Will up vote!
Chapter 9 Solutions
EBK GENERAL, ORGANIC, AND BIOLOGICAL CH
Ch. 9.1 - Prob. 1QQCh. 9.1 - Prob. 2QQCh. 9.1 - Prob. 3QQCh. 9.2 - The proper assignment of oxidation numbers to the...Ch. 9.2 - The proper assignment of oxidation numbers to the...Ch. 9.2 - Prob. 3QQCh. 9.3 - Prob. 1QQCh. 9.3 - Prob. 2QQCh. 9.3 - Prob. 3QQCh. 9.3 - Prob. 4QQ
Ch. 9.3 - Prob. 5QQCh. 9.4 - Prob. 1QQCh. 9.4 - Prob. 2QQCh. 9.4 - Prob. 3QQCh. 9.5 - Prob. 1QQCh. 9.5 - Prob. 2QQCh. 9.5 - For endothermic chemical reactions the energy...Ch. 9.6 - Prob. 1QQCh. 9.6 - Prob. 2QQCh. 9.6 - Prob. 3QQCh. 9.7 - Prob. 1QQCh. 9.7 - Prob. 2QQCh. 9.7 - Prob. 3QQCh. 9.8 - Which of the following is the correct equilibrium...Ch. 9.8 - Prob. 2QQCh. 9.8 - Prob. 3QQCh. 9.9 - Prob. 1QQCh. 9.9 - Prob. 2QQCh. 9.9 - Prob. 3QQCh. 9.9 - Prob. 4QQCh. 9 - What is the general chemical equation for each of...Ch. 9 - What is the general chemical equation for each of...Ch. 9 - Classify each of the following reactions as a...Ch. 9 - Classify each of the following reactions as a...Ch. 9 - Write the chemical formulas for the products...Ch. 9 - Write the chemical formulas for the products...Ch. 9 - Indicate whether or not each of the following...Ch. 9 - Indicate whether or not each of the following...Ch. 9 - Indicate to which of the following types of...Ch. 9 - Indicate to which of the following types of...Ch. 9 - What is the oxidation number of S in each of the...Ch. 9 - Prob. 9.12EPCh. 9 - Determine the oxidation number of the indicated...Ch. 9 - Determine the oxidation number of the indicated...Ch. 9 - Prob. 9.15EPCh. 9 - Prob. 9.16EPCh. 9 - What is the oxidation number of each element...Ch. 9 - What is the oxidation number of each element...Ch. 9 - Classify each of the following reactions as a...Ch. 9 - Classify each of the following reactions as a...Ch. 9 - Classify each of the following reactions as (1) a...Ch. 9 - Prob. 9.22EPCh. 9 - Classify each of the following reactions using one...Ch. 9 - Classify each of the following reactions using one...Ch. 9 - Prob. 9.25EPCh. 9 - In each of the following changes is the reactant...Ch. 9 - Identify which substance is oxidized and which...Ch. 9 - Identify which substance is oxidized and which...Ch. 9 - Prob. 9.29EPCh. 9 - Prob. 9.30EPCh. 9 - Indicate whether each of the following substances...Ch. 9 - Indicate whether each of the following substances...Ch. 9 - Prob. 9.33EPCh. 9 - Prob. 9.34EPCh. 9 - What are the three central concepts associated...Ch. 9 - Why are most chemical reactions carried out either...Ch. 9 - What two factors determine whether a collision...Ch. 9 - What happens to the reactants in an ineffective...Ch. 9 - Which of the following reactions are endothermic,...Ch. 9 - Prob. 9.40EPCh. 9 - Should heat be added as a reactant or as a product...Ch. 9 - Should heat be added as a reactant or as a product...Ch. 9 - Prob. 9.43EPCh. 9 - Indicate whether each of the following is a...Ch. 9 - Sketch an energy diagram graph representing an...Ch. 9 - Sketch an energy diagram graph representing an...Ch. 9 - Using collision theory, indicate why each of the...Ch. 9 - Using collision theory, indicate why each of the...Ch. 9 - Substances burn more rapidly in pure oxygen than...Ch. 9 - Milk will sour in a couple of days when left at...Ch. 9 - Will each of the changes listed increase or...Ch. 9 - Will each of the changes listed increase or...Ch. 9 - For each of the changes listed will the rate of...Ch. 9 - For each of the changes listed will the rate of...Ch. 9 - Prob. 9.55EPCh. 9 - Draw an energy diagram graph for an endothermic...Ch. 9 - The characteristics of four reactions, each of...Ch. 9 - The characteristics of four reactions, each of...Ch. 9 - What condition must be met in order for a system...Ch. 9 - What relationship exists between the rates of the...Ch. 9 - What does the term reversible reaction mean?Ch. 9 - What does the notation denote when it is used in...Ch. 9 - Consider the following equilibrium system....Ch. 9 - Consider the following equilibrium system....Ch. 9 - Prob. 9.65EPCh. 9 - Sketch a graph showing how the rates of the...Ch. 9 - The following series of diagrams represent the...Ch. 9 - The following series of diagrams represent the...Ch. 9 - For the reaction A2 + 2B 2AB, diagram I depicts...Ch. 9 - For the reaction A2 + B2 2AB, diagram I depicts...Ch. 9 - Write equilibrium constant expressions for the...Ch. 9 - Write equilibrium constant expressions for the...Ch. 9 - Write equilibrium constant expressions for the...Ch. 9 - Prob. 9.74EPCh. 9 - Calculate the value of the equilibrium constant...Ch. 9 - Calculate the value of the equilibrium constant...Ch. 9 - Prob. 9.77EPCh. 9 - Use the given Keq value and the terminology in...Ch. 9 - Write a balanced chemical equation for a totally...Ch. 9 - Write a balanced chemical equation for a totally...Ch. 9 - The following four diagrams represent gaseous...Ch. 9 - Based on the diagrams, chemical reaction, and...Ch. 9 - The following four diagrams represent gaseous...Ch. 9 - Based on the diagrams, chemical reaction, and...Ch. 9 - Indicate whether or not each of the following...Ch. 9 - Indicate whether or not each of the following...Ch. 9 - For the generalized chemical reaction...Ch. 9 - For the generalized chemical reaction...Ch. 9 - Prob. 9.89EPCh. 9 - For the reaction C6H6(g)+3H2(g)C6H12(g)+heat...Ch. 9 - Consider the following chemical system at...Ch. 9 - Prob. 9.92EPCh. 9 - The following two diagrams represent the...Ch. 9 - The following two diagrams represent the...Ch. 9 - Indicate whether or not product formation...Ch. 9 - Prob. 9.96EPCh. 9 - Prob. 9.97EPCh. 9 - Indicate whether or not product formation...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- Q1: Answer the questions for the reaction below: ..!! Br OH a) Predict the product(s) of the reaction. b) Is the substrate optically active? Are the product(s) optically active as a mix? c) Draw the curved arrow mechanism for the reaction. d) What happens to the SN1 reaction rate in each of these instances: 1. Change the substrate to Br "CI 2. Change the substrate to 3. Change the solvent from 100% CH3CH2OH to 10% CH3CH2OH + 90% DMF 4. Increase the substrate concentration by 3-fold.arrow_forwardExperiment 27 hates & Mechanisms of Reations Method I visual Clock Reaction A. Concentration effects on reaction Rates Iodine Run [I] mol/L [S₂082] | Time mo/L (SCC) 0.04 54.7 Log 1/ Time Temp Log [ ] 13,20] (time) / [I] 199 20.06 23.0 30.04 0.04 0.04 80.0 22.8 45 40.02 0.04 79.0 21.6 50.08 0.03 51.0 22.4 60-080-02 95.0 23.4 7 0.08 0-01 1970 23.4 8 0.08 0.04 16.1 22.6arrow_forward(15 pts) Consider the molecule B2H6. Generate a molecular orbital diagram but this time using a different approach that draws on your knowledge and ability to put concepts together. First use VSEPR or some other method to make sure you know the ground state structure of the molecule. Next, generate an MO diagram for BH2. Sketch the highest occupied and lowest unoccupied MOs of the BH2 fragment. These are called frontier orbitals. Now use these frontier orbitals as your basis set for producing LGO's for B2H6. Since the BH2 frontier orbitals become the LGOS, you will have to think about what is in the middle of the molecule and treat its basis as well. Do you arrive at the same qualitative MO diagram as is discussed in the book? Sketch the new highest occupied and lowest unoccupied MOs for the molecule (B2H6).arrow_forward
- Q8: Propose an efficient synthesis of cyclopentene from cyclopentane.arrow_forwardQ7: Use compound A-D, design two different ways to synthesize E. Which way is preferred? Please explain. CH3I ONa NaOCH 3 A B C D E OCH3arrow_forwardPredict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2).arrow_forward
- (10 pts) The density of metallic copper is 8.92 g cm³. The structure of this metal is cubic close-packed. What is the atomic radius of copper in copper metal?arrow_forwardPredict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2).arrow_forwardPredict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2).arrow_forward
- Q3: Rank the following compounds in increasing reactivity of E1 and E2 eliminations, respectively. Br ca. go do A CI CI B C CI Darrow_forwardQ5: Predict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2). H₂O דיי "Br KN3 CH3CH2OH NaNH2 NH3 Page 3 of 6 Chem 0310 Organic Chemistry 1 HW Problem Sets CI Br excess NaOCH 3 CH3OH Br KOC(CH3)3 DuckDuckGarrow_forwardQ4: Circle the substrate that gives a single alkene product in a E2 elimination. CI CI Br Brarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning
Chemical Equilibria and Reaction Quotients; Author: Professor Dave Explains;https://www.youtube.com/watch?v=1GiZzCzmO5Q;License: Standard YouTube License, CC-BY