
EBK GENERAL, ORGANIC, AND BIOLOGICAL CH
7th Edition
ISBN: 8220100853180
Author: STOKER
Publisher: CENGAGE L
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 9, Problem 9.70EP
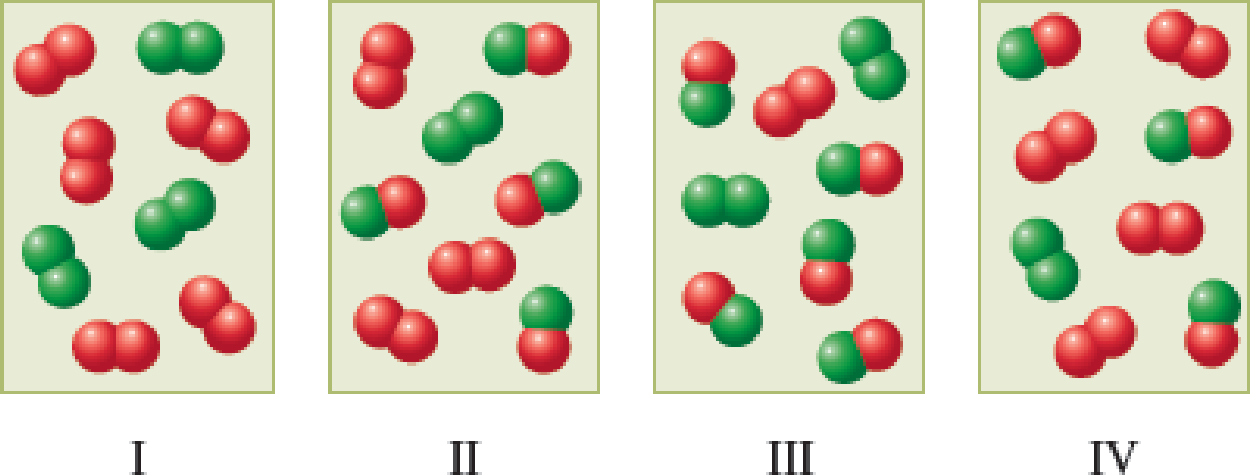
For the reaction A2 + B2 → 2AB, diagram I depicts an initial reaction mixture, where A2 molecules are red and B2 molecules are green. Which of the diagrams II through IV is a possible equilibrium state for the reaction system? There may be more than one correct answer.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Draw the stepwise mechanism for the reactions
Part I.
a)
Draw reaction mechanism for the transformations of benzophenone to benzopinacol to benzopinaco lone
b) Pinacol (2,3-dimethyl, 1-3-butanediol) on treatment w/ acid gives a mixture of pina colone
(3,3-dimethyl-2-butanone) and 2, 3-dimethyl - 1,3-butadiene. Give reasonable mechanism
the formation of
the products
For
3. The explosive decomposition of 2 mole of TNT (2,4,6-trinitrotoluene) is shown below:
Assume the C(s) is soot-basically atomic carbon (although it isn't actually atomic carbon in real life).
2
CH3
H
NO2
NO2
3N2 (g)+7CO (g) + 5H₂O (g) + 7C (s)
H
a. Use bond dissociation energies to calculate how much AU is for this reaction in kJ/mol.
Chapter 9 Solutions
EBK GENERAL, ORGANIC, AND BIOLOGICAL CH
Ch. 9.1 - Prob. 1QQCh. 9.1 - Prob. 2QQCh. 9.1 - Prob. 3QQCh. 9.2 - The proper assignment of oxidation numbers to the...Ch. 9.2 - The proper assignment of oxidation numbers to the...Ch. 9.2 - Prob. 3QQCh. 9.3 - Prob. 1QQCh. 9.3 - Prob. 2QQCh. 9.3 - Prob. 3QQCh. 9.3 - Prob. 4QQ
Ch. 9.3 - Prob. 5QQCh. 9.4 - Prob. 1QQCh. 9.4 - Prob. 2QQCh. 9.4 - Prob. 3QQCh. 9.5 - Prob. 1QQCh. 9.5 - Prob. 2QQCh. 9.5 - For endothermic chemical reactions the energy...Ch. 9.6 - Prob. 1QQCh. 9.6 - Prob. 2QQCh. 9.6 - Prob. 3QQCh. 9.7 - Prob. 1QQCh. 9.7 - Prob. 2QQCh. 9.7 - Prob. 3QQCh. 9.8 - Which of the following is the correct equilibrium...Ch. 9.8 - Prob. 2QQCh. 9.8 - Prob. 3QQCh. 9.9 - Prob. 1QQCh. 9.9 - Prob. 2QQCh. 9.9 - Prob. 3QQCh. 9.9 - Prob. 4QQCh. 9 - What is the general chemical equation for each of...Ch. 9 - What is the general chemical equation for each of...Ch. 9 - Classify each of the following reactions as a...Ch. 9 - Classify each of the following reactions as a...Ch. 9 - Write the chemical formulas for the products...Ch. 9 - Write the chemical formulas for the products...Ch. 9 - Indicate whether or not each of the following...Ch. 9 - Indicate whether or not each of the following...Ch. 9 - Indicate to which of the following types of...Ch. 9 - Indicate to which of the following types of...Ch. 9 - What is the oxidation number of S in each of the...Ch. 9 - Prob. 9.12EPCh. 9 - Determine the oxidation number of the indicated...Ch. 9 - Determine the oxidation number of the indicated...Ch. 9 - Prob. 9.15EPCh. 9 - Prob. 9.16EPCh. 9 - What is the oxidation number of each element...Ch. 9 - What is the oxidation number of each element...Ch. 9 - Classify each of the following reactions as a...Ch. 9 - Classify each of the following reactions as a...Ch. 9 - Classify each of the following reactions as (1) a...Ch. 9 - Prob. 9.22EPCh. 9 - Classify each of the following reactions using one...Ch. 9 - Classify each of the following reactions using one...Ch. 9 - Prob. 9.25EPCh. 9 - In each of the following changes is the reactant...Ch. 9 - Identify which substance is oxidized and which...Ch. 9 - Identify which substance is oxidized and which...Ch. 9 - Prob. 9.29EPCh. 9 - Prob. 9.30EPCh. 9 - Indicate whether each of the following substances...Ch. 9 - Indicate whether each of the following substances...Ch. 9 - Prob. 9.33EPCh. 9 - Prob. 9.34EPCh. 9 - What are the three central concepts associated...Ch. 9 - Why are most chemical reactions carried out either...Ch. 9 - What two factors determine whether a collision...Ch. 9 - What happens to the reactants in an ineffective...Ch. 9 - Which of the following reactions are endothermic,...Ch. 9 - Prob. 9.40EPCh. 9 - Should heat be added as a reactant or as a product...Ch. 9 - Should heat be added as a reactant or as a product...Ch. 9 - Prob. 9.43EPCh. 9 - Indicate whether each of the following is a...Ch. 9 - Sketch an energy diagram graph representing an...Ch. 9 - Sketch an energy diagram graph representing an...Ch. 9 - Using collision theory, indicate why each of the...Ch. 9 - Using collision theory, indicate why each of the...Ch. 9 - Substances burn more rapidly in pure oxygen than...Ch. 9 - Milk will sour in a couple of days when left at...Ch. 9 - Will each of the changes listed increase or...Ch. 9 - Will each of the changes listed increase or...Ch. 9 - For each of the changes listed will the rate of...Ch. 9 - For each of the changes listed will the rate of...Ch. 9 - Prob. 9.55EPCh. 9 - Draw an energy diagram graph for an endothermic...Ch. 9 - The characteristics of four reactions, each of...Ch. 9 - The characteristics of four reactions, each of...Ch. 9 - What condition must be met in order for a system...Ch. 9 - What relationship exists between the rates of the...Ch. 9 - What does the term reversible reaction mean?Ch. 9 - What does the notation denote when it is used in...Ch. 9 - Consider the following equilibrium system....Ch. 9 - Consider the following equilibrium system....Ch. 9 - Prob. 9.65EPCh. 9 - Sketch a graph showing how the rates of the...Ch. 9 - The following series of diagrams represent the...Ch. 9 - The following series of diagrams represent the...Ch. 9 - For the reaction A2 + 2B 2AB, diagram I depicts...Ch. 9 - For the reaction A2 + B2 2AB, diagram I depicts...Ch. 9 - Write equilibrium constant expressions for the...Ch. 9 - Write equilibrium constant expressions for the...Ch. 9 - Write equilibrium constant expressions for the...Ch. 9 - Prob. 9.74EPCh. 9 - Calculate the value of the equilibrium constant...Ch. 9 - Calculate the value of the equilibrium constant...Ch. 9 - Prob. 9.77EPCh. 9 - Use the given Keq value and the terminology in...Ch. 9 - Write a balanced chemical equation for a totally...Ch. 9 - Write a balanced chemical equation for a totally...Ch. 9 - The following four diagrams represent gaseous...Ch. 9 - Based on the diagrams, chemical reaction, and...Ch. 9 - The following four diagrams represent gaseous...Ch. 9 - Based on the diagrams, chemical reaction, and...Ch. 9 - Indicate whether or not each of the following...Ch. 9 - Indicate whether or not each of the following...Ch. 9 - For the generalized chemical reaction...Ch. 9 - For the generalized chemical reaction...Ch. 9 - Prob. 9.89EPCh. 9 - For the reaction C6H6(g)+3H2(g)C6H12(g)+heat...Ch. 9 - Consider the following chemical system at...Ch. 9 - Prob. 9.92EPCh. 9 - The following two diagrams represent the...Ch. 9 - The following two diagrams represent the...Ch. 9 - Indicate whether or not product formation...Ch. 9 - Prob. 9.96EPCh. 9 - Prob. 9.97EPCh. 9 - Indicate whether or not product formation...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- Part I. Draw reaction mechanism for the transformations of benzophenone to benzopinacol to benzopinaco lone and answer the ff: Pinacol (2,3-dimethyl, 1-3-butanediol) on treatment w/ acid gives a mixture of pina colone and (3,3-dimethyl-2-butanone) 2,3-dimethyl-1,3-butadiene. Give reasonable mechanism the formation of the products Forarrow_forwardShow the mechanism for these reactionsarrow_forwardDraw the stepwise mechanismarrow_forward
- Draw a structural formula of the principal product formed when benzonitrile is treated with each reagent. (a) H₂O (one equivalent), H₂SO₄, heat (b) H₂O (excess), H₂SO₄, heat (c) NaOH, H₂O, heat (d) LiAlH4, then H₂Oarrow_forwardDraw the stepwise mechanism for the reactionsarrow_forwardDraw stepwise mechanismarrow_forward
- Part I. Draw reaction mechanism for the transformations of benzophenone to benzopinacol to benzopinaco lone and answer the ff: a) Give the major reason for the exposure of benzophenone al isopropyl alcohol (w/acid) to direct sunlight of pina colone Mechanism For b) Pinacol (2,3-dimethy 1, 1-3-butanediol) on treatment w/ acid gives a mixture (3,3-dimethyl-2-butanone) and 2, 3-dimethyl-1,3-butadiene. Give reasonable the formation of the productsarrow_forwardwhat are the Iupac names for each structurearrow_forwardWhat are the IUPAC Names of all the compounds in the picture?arrow_forward
- 1) a) Give the dominant Intermolecular Force (IMF) in a sample of each of the following compounds. Please show your work. (8) SF2, CH,OH, C₂H₂ b) Based on your answers given above, list the compounds in order of their Boiling Point from low to high. (8)arrow_forward19.78 Write the products of the following sequences of reactions. Refer to your reaction road- maps to see how the combined reactions allow you to "navigate" between the different functional groups. Note that you will need your old Chapters 6-11 and Chapters 15-18 roadmaps along with your new Chapter 19 roadmap for these. (a) 1. BHS 2. H₂O₂ 3. H₂CrO4 4. SOCI₂ (b) 1. Cl₂/hv 2. KOLBU 3. H₂O, catalytic H₂SO4 4. H₂CrO4 Reaction Roadmap An alkene 5. EtOH 6.0.5 Equiv. NaOEt/EtOH 7. Mild H₂O An alkane 1.0 2. (CH3)₂S 3. H₂CrO (d) (c) 4. Excess EtOH, catalytic H₂SO OH 4. Mild H₂O* 5.0.5 Equiv. NaOEt/EtOH An alkene 6. Mild H₂O* A carboxylic acid 7. Mild H₂O* 1. SOC₁₂ 2. EtOH 3.0.5 Equiv. NaOEt/E:OH 5.1.0 Equiv. NaOEt 6. NH₂ (e) 1. 0.5 Equiv. NaOEt/EtOH 2. Mild H₂O* Br (f) i H An aldehyde 1. Catalytic NaOE/EtOH 2. H₂O*, heat 3. (CH,CH₂)₂Culi 4. Mild H₂O* 5.1.0 Equiv. LDA Br An ester 4. NaOH, H₂O 5. Mild H₂O* 6. Heat 7. MgBr 8. Mild H₂O* 7. Mild H₂O+arrow_forwardLi+ is a hard acid. With this in mind, which if the following compounds should be most soluble in water? Group of answer choices LiBr LiI LiF LiClarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning
Chemical Equilibria and Reaction Quotients; Author: Professor Dave Explains;https://www.youtube.com/watch?v=1GiZzCzmO5Q;License: Standard YouTube License, CC-BY