
(Flame retardants) Hydrogen radicals are important to sustaining combustion reactions. Consequently, if chemical compounds that can scavenge the hydrogen radicals are introduced, the flames can be extinguished. While many reactions occur during the combustion process, we shall choose CO flames as a model system to illustrate the process (S. Senkan et al., Combustion and Flame, 69, 113). In the absence of inhibitors
The last two reactions are rapid compared to the first two. When HCl is introduced to the flame, the following additional reactions occur:
Assume that all reactions are elementary and that the PSSH holds for the O·, OH·, and Cl· radicals.
- (a) Derive a rate law for the consumption of CO when no retardant is present.
- (b) Derive an equation for the concentration of H· as a function of time, assuming constant concentration of O2, CO, and H2O for both uninhibited combustion and combustion with HCl present. Sketch H· versus time for both cases.
(a)
Interpretation:
Rate law for the consumption of
Concept Introduction:
Rate law or rate equation: Rate law:
It is generally the rate equation that consists of the reaction rate with the concentration or the pressures of the reactants and constant parameters.
Explanation of Solution
Uninhibited combustion can be given as:
Reaction when
Rate law for the consumption of
(b)
Interpretation:
Equation for the concentration of
Concept Introduction:
Rate law or rate equation: Rate law:
It is generally the rate equation that consists of the reaction rate with the concentration or the pressures of the reactants and constant parameters.
Explanation of Solution
Uninhibited combustion can be given as:
Reaction when
Rate law for the consumption of
Rate of formation of active intermediates is zero. Here,
Hence,
Also,
Substitute
Therefore,
Substitute equation for
When volume is constant, concentration of
By integration factor
When
Therefore,
When
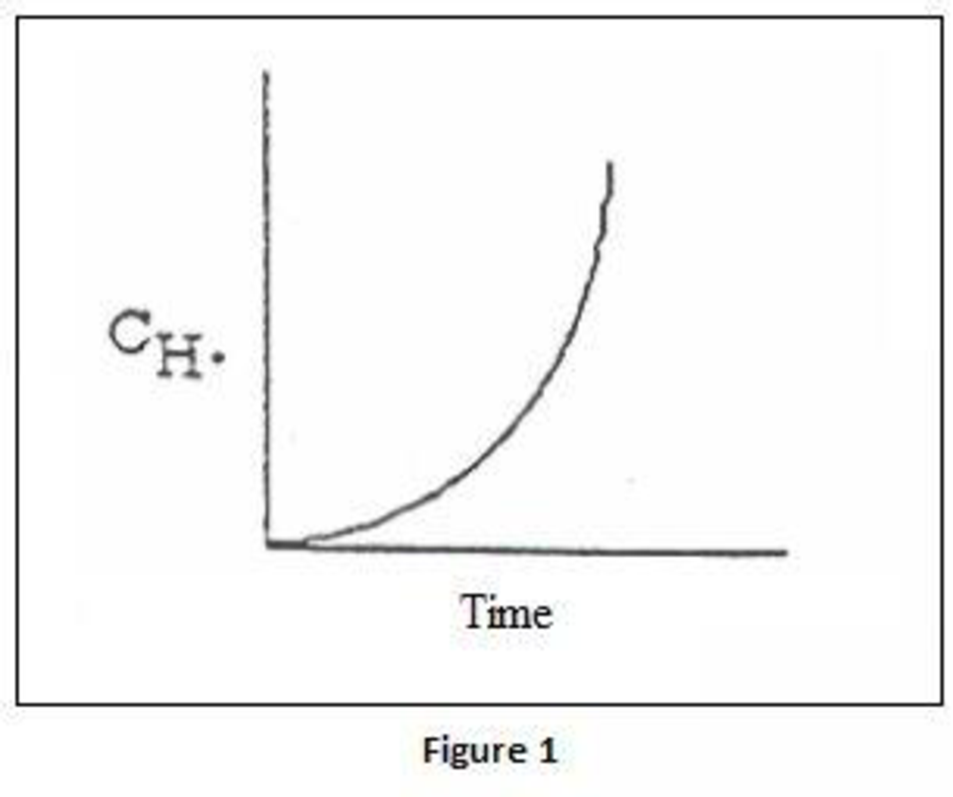
When
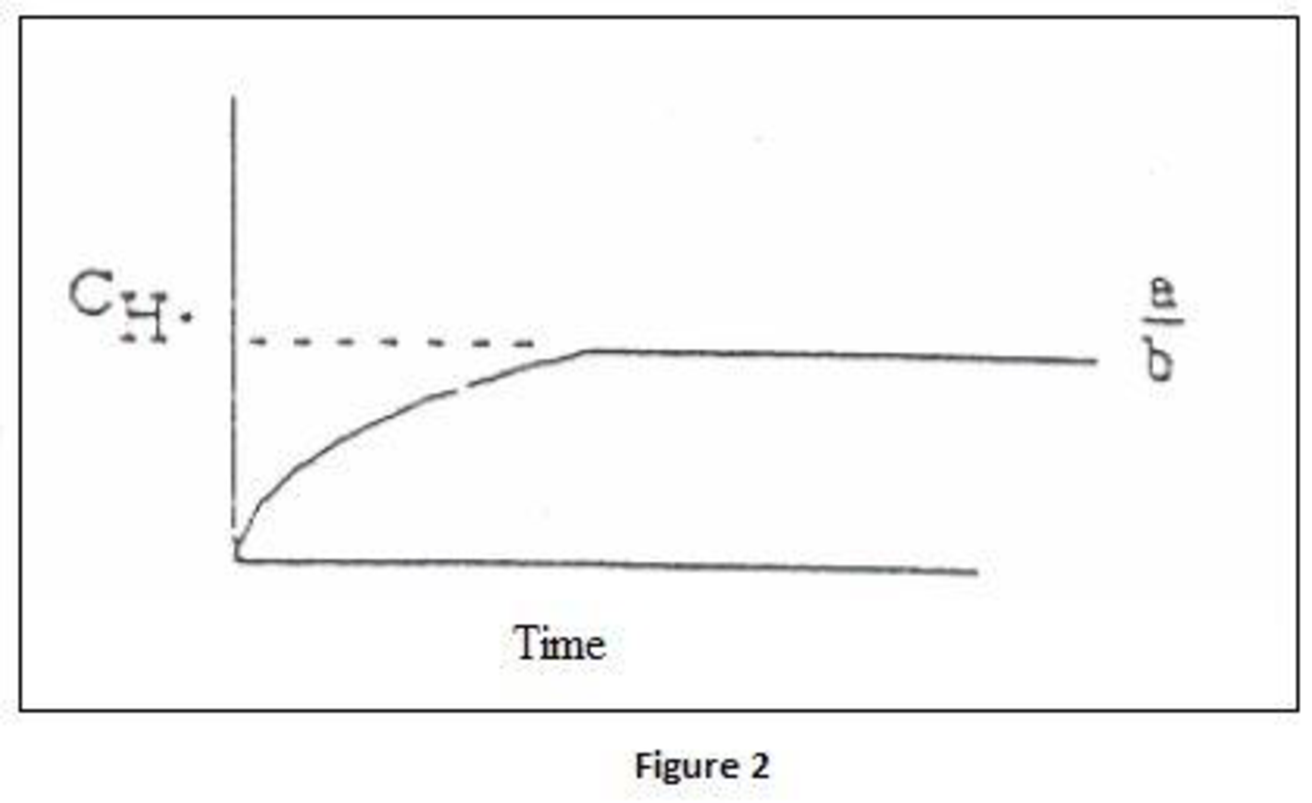
Concentration of
Where,
When
Therefore,
Want to see more full solutions like this?
Chapter 9 Solutions
Elements of Chemical Reaction Engineering (5th Edition) (Prentice Hall International Series in the Physical and Chemical Engineering Sciences)
Additional Engineering Textbook Solutions
Degarmo's Materials And Processes In Manufacturing
Starting Out With Visual Basic (8th Edition)
Starting Out with Python (4th Edition)
Mechanics of Materials (10th Edition)
Thinking Like an Engineer: An Active Learning Approach (4th Edition)
Java How to Program, Early Objects (11th Edition) (Deitel: How to Program)
- heat and mass transferarrow_forwardheat and mass transferarrow_forwardA biodiesel mixture consisting of 60 mol% methyl oleate (MO), 25 mol% methyl linoleate (ML), and 15 mol% methyl palmitate (MP) is held at 373.15 K and 200 MPa. Given PC-SAFT parameters: segment number \( m_i = [5.7, 6.3, 4.8] \), segment diameter \( \sigma_i = [3.95, 3.98, 3.91] \) Å, dispersion energy \( \epsilon_i/k = [260, 270, 250] \) K, and binary interaction parameters \( k_{ij} = 0.01 \), determine the isentropic speed of sound (m/s) using the PC-SAFT Helmholtz energy formulation and the thermodynamic identity\[c^2 = \left( \frac{\partial P}{\partial \rho} \right)_T + \frac{T \left( \frac{\partial P}{\partial T} \right)_\rho^2 }{ \rho^2 c_v },\]assuming the density is precomputed at 200 MPa and \( c_v \) is obtained from ideal mixing of pure-component values.arrow_forward
- A steady Williamson nanofluid containing Cu nanoparticles flows over a permeable wedge with wall suction \( V_w = 0.015 \, \text{m/s} \), under a transverse magnetic field \( B_0 = 0.6 \, \text{T} \). The flow obeys the Buongiorno model, with \( D_B = 9 \times 10^{-10} \), \( D_T = 3.5 \times 10^{-8} \), and activation energy \( E_a = 65 \times 10^3 \). Hall and ion-slip effects are included with \( m_e = 0.4 \), \( \beta = 0.15 \). Thermal conductivity varies as \( k(T) = 0.6 (1 + 0.002 (T - 305)) \). Apply velocity and thermal jump conditions with \( \alpha_u = 0.9 \), \( \alpha_T = 0.8 \), \( \lambda = 2 \times 10^{-7} \). Using Keller’s method and similarity variables for wedge parameter \( m = 0.4 \), determine the entropy generation number \( N_s \) at \( x = 0.03 \), where \[N_s = \frac{k(T)}{T_\infty^2} \left( \frac{\partial T}{\partial y} \right)^2 + \frac{\mu}{T_\infty} \left( \frac{\partial u}{\partial y} \right)^2.\]arrow_forwardE. coli was continuously cultured in a continuous stirred tank fermenter with a working volume of 1 L by chemostat. A medium containing 4.0 g/L of glucose as a carbon source was fed to the fermenter at a constant flow rate of 0.5 L/hr, and the glucose concentration in the output stream was 0.20 g/L. The cell yield with respect to glucose was 0.42 g dry cells per gram glucose.arrow_forwardPlease do not use any AI tools to solve this question. I need a fully manual, step-by-step solution with clear explanations, as if it were done by a human tutor. No AI-generated responses, please.arrow_forward
- In the published paper, "Exergy-based Greenhouse gas metric of buildings", use the value of the Exergy Loss of Emission of carbon dioxide to evaluate the index value for 1000 occupants for 50 years building life span in kilogram per person per yeararrow_forwardA CO₂-saturated brine (ionic strength = 2.5 mol/kg, pH = 3, a_H⁺ = 0.01) flows at 2 cm/s through a horizontal tubular reactor (L = 1 m, D = 0.05 m) packed with 5 kg of olivine (Zhuravlev-BET surface area = 30 m²/g). The system operates at 90 °C and 40 bar, and external mass transfer resistance is negligible. The rate-limiting step is electron transfer at the mineral surface, governed by Marcus theory, with λ = 0.75 eV, ΔG° = –0.30 eV, and k₀ = 10⁶ s⁻¹. The Mg²⁺ activity coefficient is γ = 0.76 (from PHREEQC with Pitzer model). For Mg₂SiO₄ + 4 H⁺ → 2 Mg²⁺ + SiO₂(aq) + 2 H₂O, determine the total moles of Mg²⁺ released after 10 minutes at steady state.arrow_forwardIn baseball, batters frequently attempt to hit a ball as far as possible. However, baseballs are inelastic with an officially required "coefficient of restitution" CR ≈ 0.55 on ash wood. The coefficient of restitution of a dropped ball iswhere H is the initial drop height, h is the max. height on the rebound, and h ≈ 2πH tan δ for a homogeneous material. Assuming that a baseball is homogeneous and has a storage modulus approximately the same as that of cork (E' = 18.6 MPa), what must the value of the loss modulus E'' be so that the ball is regulation?arrow_forward
- The creep strain rate of a polymer (in “Hz”) is given by where T is the temperature and Q = 100. kJ/mol is the activation energy. How long t will it take for a rod of this polymer to extend from 10. mm to 15 mm at 100. °C?arrow_forwardCreep compliance J(t) An amorphous polymer has Tg = 100 °C. A creep modulus of 1/J = 1 GPa was measured after t₁ = 1 h at T₁ = 90 °C. Suppose that log 10 a (T) = 17.5(T-Tg) 52+(T-Tg) 1 for this material. What is the shift factor a at T = T₁ relative to the reference Tg? What is the time t₂ required to reach a modulus of 1 GPa at T2 = 80 °C? TR = T = 100 °C |J(t) = 1 GPa-1 + log a(T₁) T₁ = 75 °C T₂ = 50 °C log tr log t₁ log t log t₂ = ?arrow_forwardA 0.45 mol/kg aqueous solution of 3-(methylamino)propylamine and 1-methylpiperazine (1:1 molar) flows at 0.5 g/s through a 2.5 m horizontal stainless steel coil (inner diameter 1.2 mm), entering at 358.15 K and 22 MPa and exiting at 20 MPa. A constant wall heat flux of 28 W is applied. Local density and isobaric heat capacity are obtained through from the Benedict–Webb–Rubin equation of state, with a 3% increase in heat capacity to account for wall sorption. Dynamic viscosity is obtained using the Green-Kubo relation with a given integral value of 2.1 × 10⁻¹⁰ Pa².s, and thermal conductivity is assumed constant. The local Nusselt number is corrected for thermal development using Nu(x) = 3.66 + (0.065 · Gz(x)^0.7) / (1 + 0.04 · Gz(x)^0.7), where Gz(x) = D · Re(x) · Pr(x) / x. Through a spatially resolved numerical integration of the 1D steady-state energy equation and determine the outlet temperature (K).arrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





