
Chemistry (OER)
2nd Edition
ISBN: 9781947172616
Author: OpenStax
Publisher: OpenStax College
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 9, Problem 93E
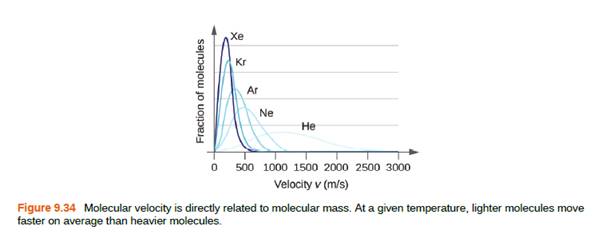
The distribution of molecular velocities in a sample of helium is shown in Figure 9.34. If the sample is cooled, will the distribution of velocities look more like that of H2 or of H2O? Explain your answer.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Briefly describe the structure and bonding of graphite. Indicate some type of inorganic compound with a complex structure that forms graphite.
For c4h5n2 draw the lewis dot structure
Indicate the coordination forms of Si in silicates.
Chapter 9 Solutions
Chemistry (OER)
Ch. 9 - Why are sharp knives more effective than dull...Ch. 9 - Why do some small bridges have weight limits that...Ch. 9 - Why should you roll or belly-crawl rather than...Ch. 9 - A typical barometric pressure in Redding....Ch. 9 - A typical barometric pressure in Denver, Colorado,...Ch. 9 - A typical barometric pressure in Kansas City is...Ch. 9 - Canadian tire pressure gauges are marked in units...Ch. 9 - Dining the Viking landings on Mars, the...Ch. 9 - The pressure of the atmosphere on the surface of...Ch. 9 - A medical laboratory catalog describes the...
Ch. 9 - Consider this scenario and answer the following...Ch. 9 - Why is it necessary to use a nonvolatile liquid in...Ch. 9 - The pressure of a sample of gas is measured at sea...Ch. 9 - The pressure of a sample of gas is measured with...Ch. 9 - The pressure of a sample of gas is measured at sea...Ch. 9 - The pressure of a sample of gas ¡s measured a sea...Ch. 9 - How would the use of a volatile liquid affect the...Ch. 9 - Sometimes leaving a bicycle in the sun on a hot...Ch. 9 - Explain how the volume of the bubbles exhausted by...Ch. 9 - One way to state Boyle’s law is All other things...Ch. 9 - An alternate way to state Avogadro’s law is A1l...Ch. 9 - How would the graph in Figure 9.12 change if the...Ch. 9 - How would the graph in Figure 9.13 change if the...Ch. 9 - In addition to the data found in Figure 9.13, what...Ch. 9 - Determine the volume of 1 mol of CH4 gas at 150 K...Ch. 9 - Determine the pressure of the gas in the syringe...Ch. 9 - A spray can is used until it is empty except for...Ch. 9 - What is the temperature of an 11.2-L sample of...Ch. 9 - À 2.50-L volume of hydrogen measured at —196 C is...Ch. 9 - A balloon inflated with three breaths of air has a...Ch. 9 - A weather balloon contains 8.80 moles of helium at...Ch. 9 - The volume of an automobile air bag was 66.8 L...Ch. 9 - How many moles of gaseous boron trifluoride, BF3,...Ch. 9 - Iodine, I2, is a solid at room temperature but...Ch. 9 - How many grams of gas are present in each of the...Ch. 9 - A high altitude balloon is filled with 1041104 L...Ch. 9 - A cylinder of medical oxygen has a volume of 3S.4...Ch. 9 - A large scuba tank (Figure 9.16) with a volume of...Ch. 9 - A 20.0-L cylinder containing 11.34 kg of butane,...Ch. 9 - While resting, the average 70-kg human male...Ch. 9 - For a given amount of gas showing ideal behavior,...Ch. 9 - A liter of methane gas, CH4, at STP contains more...Ch. 9 - The effect of chlorofluorocarbons (such as CCl2F2)...Ch. 9 - As 1 g of (lie radioactive element radium decays...Ch. 9 - A balloon that is 100.21 L at 21 C and 0.981 atm...Ch. 9 - If the temperature of a fixed amount of a gas is...Ch. 9 - If the volume of a fixed amount of a gas is...Ch. 9 - What is the density of laughing gas, dinitrogen...Ch. 9 - Calculate the density of Freon 12, CF2Cl2, at 30.0...Ch. 9 - Which is denser at the same temperature and...Ch. 9 - A cylinder of O2(g) used in breathing by emphysema...Ch. 9 - What is the molar mass of a gas if 0.0494 g of the...Ch. 9 - What is the molar mass of a gas if 0.281 g of the...Ch. 9 - How could you show experimentally that the...Ch. 9 - The density of a certain gaseous fluoride of...Ch. 9 - Consider this question: What is the molecular...Ch. 9 - A 36.0—L cylinder of a gas used for calibration of...Ch. 9 - A cylinder of a gas mixture used for calibration...Ch. 9 - A sample of gas isolated from unrefined petroleum...Ch. 9 - A mixture of 0.200 g of 1.00 g of and 0.820 g of...Ch. 9 - Most mixtures of hydrogen gas with oxygen gas are...Ch. 9 - A commercial mercury vapor analyzer can detect in...Ch. 9 - A sample of carbon monoxide was collected over...Ch. 9 - In an experiment in a general chemistry...Ch. 9 - Joseph Priestley first prepared pure oxygen by...Ch. 9 - Cavendish prepared hydrogen in 176G by the novel...Ch. 9 - The chlorofluorocarbon CCl2F2 can be recycled into...Ch. 9 - Automobile air bags are inflated with nitrogen...Ch. 9 - Lime, CaO, is produced by heating calcium...Ch. 9 - Before small batteries were available, carbide...Ch. 9 - Calculate the volume of oxygen required to burn...Ch. 9 - What volume of O2 at STP is required to oxidize...Ch. 9 - Consider the following questions: (a) What is the...Ch. 9 - Methanol, CH3OH, is produced industrially by the...Ch. 9 - What volume of oxygen a 423.0 K and a pressure of...Ch. 9 - A 230-L sample of a colorless gas at STP...Ch. 9 - Ethanol, C2H5OH, is produced industrially from...Ch. 9 - One molecule of hemoglobin will combine with four...Ch. 9 - A sample of a compound of xenon and fluorine was...Ch. 9 - One method of analyzing amino acids is the van...Ch. 9 - A balloon filled with helium gas is found to take...Ch. 9 - Explain why the numbers of molecules are not...Ch. 9 - Starting with the definition of rate of effusion...Ch. 9 - Heavy water, D2O (molar mass = 20.03 g mol-1). can...Ch. 9 - Which of the following gases diffuse more slowly...Ch. 9 - During the discussion of gaseous diffusion for...Ch. 9 - Calculate the relative rate of diffusion of 1H2...Ch. 9 - A gas of unknown identity diffuses at a rate of...Ch. 9 - When two cotton plugs. one moistened with ammonia...Ch. 9 - Using the postulates of the kinetic molecular...Ch. 9 - Can the speed of a given molecule in a gas double...Ch. 9 - Describe what happens o the average kinetic energy...Ch. 9 - The distribution of molecular velocities in a...Ch. 9 - What is the ratio of the average kinetic energy of...Ch. 9 - A 1-L sample of CO initially at STP is heated to...Ch. 9 - The root mean square speed of H2, molecules at 25...Ch. 9 - Answer the following questions: (a) Is the...Ch. 9 - Show that the ratio of the rate of diffusion of...Ch. 9 - Graphs showing the behavior of several different...Ch. 9 - Explain why the plot of PV for CO2 differs from...Ch. 9 - Under which of the following sets of conditions...Ch. 9 - Describe the factors responsible for the deviation...Ch. 9 - For which of the following gases should the...Ch. 9 - A 0.245-L flask contains 0.467 mol CO2 at 159 C....Ch. 9 - Answer the following questions: (a) If XX behaved...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Q5. Which compound is ionic?
a.
b.
c.
d.
Introductory Chemistry (6th Edition)
17. Anthropologists are interested in locating areas in Africa where fossils 4-8 million years old might be fou...
Campbell Biology: Concepts & Connections (9th Edition)
[14.110] The following mechanism has been proposed for the gas-phase reaction of chloroform (CHCI3) and chlorin...
Chemistry: The Central Science (14th Edition)
CAUTION Why does the presence of extinct forms and transitional features in the fossil record support the patte...
Biological Science (6th Edition)
4. What five specific threats to biodiversity are described in this chapter? Provide an example of each.
Biology: Life on Earth (11th Edition)
If someone at the other end of a room smokes a cigarette, you may breathe in some smoke. The movement of smoke ...
Campbell Essential Biology with Physiology (5th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Briefly indicate the structure and bonding of silicates.arrow_forward4 Part C Give the IUPAC name and a common name for the following ether: Spell out the full names of the compound in the indicated order separated by a comma.arrow_forwardTry: Draw possible resonance contributing structures for the following organic species: CH3CH2NO2 [CH2CHCH2] [CH2CHCHO] [CH2CHCH2] [CH2CHNH2]arrow_forward
- Complete the following synthesis. (d). H+ ง сarrow_forwardCan the target compound be efficiently synthesized in good yield from the substituted benzene of the starting material? If yes, draw the synthesis. Include all steps and all reactants.arrow_forwardThis is a synthesis question. Why is this method wrong or worse than the "correct" method? You could do it thiss way, couldn't you?arrow_forward
- Try: Draw the best Lewis structure showing all non-bonding electrons and all formal charges if any: (CH3)3CCNO NCO- HN3 [CH3OH2]*arrow_forwardWhat are the major products of the following reaction? Draw all the major products. If there are no major products, then there is no reaction that will take place. Use wedge and dash bonds when necessary.arrow_forwardZeolites. State their composition and structure. Give an example.arrow_forward
- Don't used hand raiting and show all reactionsarrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forwardIX) By writing the appropriate electron configurations and orbital box diagrams briefly EXPLAIN in your own words each one of the following questions: a) The bond length of the Br2 molecule is 2.28 Å, while the bond length of the compound KBr is 3.34 Å. The radius of K✶ is 1.52 Å. Determine the atomic radius in Å of the bromine atom and of the bromide ion. Br = Br b) Explain why there is a large difference in the atomic sizes or radius of the two (Br and Br). Tarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning