
Organic And Biological Chemistry
7th Edition
ISBN: 9781305081079
Author: STOKER, H. Stephen (howard Stephen)
Publisher: Cengage Learning,
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 9, Problem 9.10EP
How many carbon atoms are present in the R group in each of the following standard amino acids?
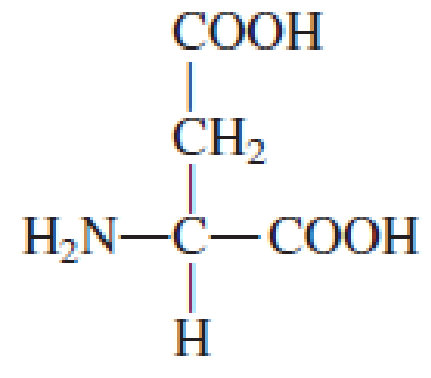
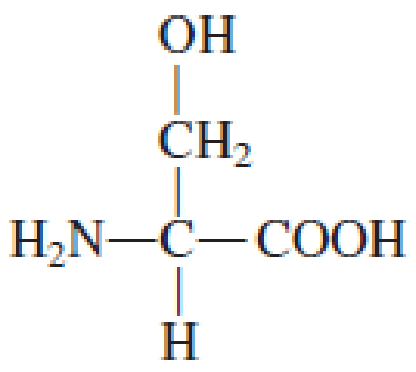
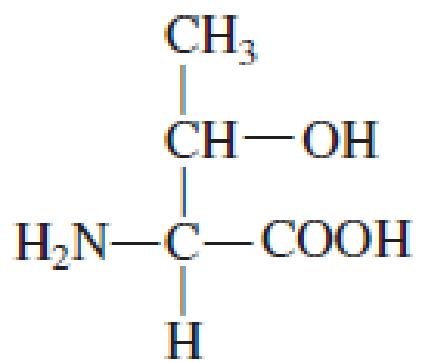
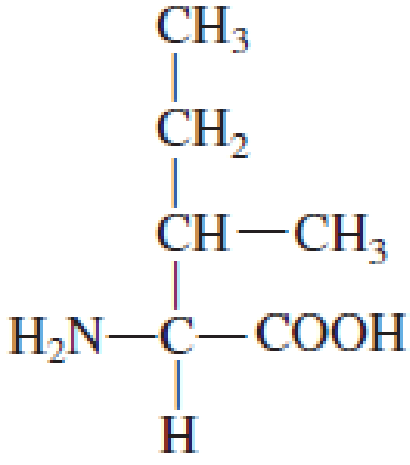
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
For the condensation reaction between Alanine and histidine write the amididation reaction mechanism using arrows then write the three letter code for the product of the reaction and the one letter code for the product of the reaction.
Write the amididation reaction mechanism of p-aminophenol and acetic acid to produce acetaminophen please use arrows.
Name the following using IUPAC.
Chapter 9 Solutions
Organic And Biological Chemistry
Ch. 9.1 - Prob. 1QQCh. 9.1 - Proteins are naturally occurring unbranched...Ch. 9.2 - Prob. 1QQCh. 9.2 - How do the various standard amino acids differ...Ch. 9.2 - The number of carboxyl groups and amino groups...Ch. 9.2 - How many different subclassifications are there...Ch. 9.2 - Which of the following statements concerning...Ch. 9.3 - Prob. 1QQCh. 9.3 - Proteins from plant sources are a. always complete...Ch. 9.3 - Incomplete dietary proteins contain inadequate...
Ch. 9.4 - Which of the following statements concerning the...Ch. 9.4 - Prob. 2QQCh. 9.4 - Which of the following statements concerning...Ch. 9.5 - Which of the standard amino acids exist as...Ch. 9.5 - Which of the following is the zwitterion ion...Ch. 9.5 - Which of the following is the structural form for...Ch. 9.6 - Cysteine is unique among standard amino acids in...Ch. 9.6 - Prob. 2QQCh. 9.7 - The joining together of two amino acids to form a...Ch. 9.7 - Prob. 2QQCh. 9.7 - Which of the following statements concerning the...Ch. 9.7 - What functional group is present in the bond...Ch. 9.7 - How many isomeric tripeptides can be formed from...Ch. 9.8 - The two best-known peptide hormones present in the...Ch. 9.8 - Which of the following peptides is an important...Ch. 9.9 - The term protein is generally reserved for...Ch. 9.9 - The presence of which of the following is a...Ch. 9.9 - Which of the following is not a distinguishing...Ch. 9.10 - Prob. 1QQCh. 9.10 - Prob. 2QQCh. 9.10 - Two different proteins that contain the same...Ch. 9.11 - Prob. 1QQCh. 9.11 - Prob. 2QQCh. 9.11 - Which of the following statements concerning the ...Ch. 9.12 - Interactions between amino acid R groups are...Ch. 9.12 - Prob. 2QQCh. 9.12 - R group interactions between which of the...Ch. 9.13 - Prob. 1QQCh. 9.13 - Prob. 2QQCh. 9.14 - The complete hydrolysis of a protein produces a...Ch. 9.14 - Which of the following statements concerning...Ch. 9.15 - Which of the following levels of protein structure...Ch. 9.15 - Which of the following does not involve protein...Ch. 9.15 - Which of the following is not a denaturing agent...Ch. 9.16 - Which of the following statements concerning...Ch. 9.16 - Prob. 2QQCh. 9.16 - Prob. 3QQCh. 9.16 - In which of the following pairs of proteins are...Ch. 9.17 - Insulin and human growth hormone are examples of...Ch. 9.17 - Myoglobin and transferrin are examples of a....Ch. 9.17 - Prob. 3QQCh. 9.18 - Prob. 1QQCh. 9.18 - Which of the following statements concerning basic...Ch. 9.18 - Which of the following statements about antibodies...Ch. 9.19 - Prob. 1QQCh. 9.19 - Prob. 2QQCh. 9.19 - In which of the following pairs of plasma...Ch. 9 - Prob. 9.1EPCh. 9 - What element is always present in proteins that is...Ch. 9 - What percent of a cells overall mass is accounted...Ch. 9 - Prob. 9.4EPCh. 9 - What is signified when an amino acid is designated...Ch. 9 - Prob. 9.6EPCh. 9 - Prob. 9.7EPCh. 9 - Indicate whether or not each of the following...Ch. 9 - Prob. 9.9EPCh. 9 - How many carbon atoms are present in the R group...Ch. 9 - Prob. 9.11EPCh. 9 - Prob. 9.12EPCh. 9 - Prob. 9.13EPCh. 9 - Prob. 9.14EPCh. 9 - Prob. 9.15EPCh. 9 - Prob. 9.16EPCh. 9 - Prob. 9.17EPCh. 9 - Prob. 9.18EPCh. 9 - Prob. 9.19EPCh. 9 - Prob. 9.20EPCh. 9 - Prob. 9.21EPCh. 9 - Prob. 9.22EPCh. 9 - In what way is the structure of the amino acid...Ch. 9 - Which two of the standard amino acids are...Ch. 9 - Prob. 9.25EPCh. 9 - Prob. 9.26EPCh. 9 - Indicate whether or not each of the following...Ch. 9 - Prob. 9.28EPCh. 9 - Prob. 9.29EPCh. 9 - Prob. 9.30EPCh. 9 - Prob. 9.31EPCh. 9 - Prob. 9.32EPCh. 9 - Prob. 9.33EPCh. 9 - Prob. 9.34EPCh. 9 - To which family of mirror-image isomers do nearly...Ch. 9 - In what way is the structure of glycine different...Ch. 9 - Draw Fischer projection formulas for the following...Ch. 9 - Draw Fischer projection formulas for the following...Ch. 9 - Prob. 9.39EPCh. 9 - Prob. 9.40EPCh. 9 - At room temperature, amino acids are solids with...Ch. 9 - At room temperature, most amino acids are not very...Ch. 9 - Draw the zwitterion structure for each of the...Ch. 9 - Draw the zwitterion structure for each of the...Ch. 9 - Draw the structure of serine at each of the...Ch. 9 - Prob. 9.46EPCh. 9 - Prob. 9.47EPCh. 9 - Most amino acids have isoelectric points between...Ch. 9 - Glutamic acid exists in two low-pH forms instead...Ch. 9 - Arginine exists in two high-pH forms instead of...Ch. 9 - Prob. 9.51EPCh. 9 - In a high-pH aqueous solution, indicate whether...Ch. 9 - When two cysteine molecules dimerize, what happens...Ch. 9 - What chemical reaction involving the cysteine...Ch. 9 - What two functional groups are involved in the...Ch. 9 - Prob. 9.56EPCh. 9 - Prob. 9.57EPCh. 9 - Prob. 9.58EPCh. 9 - Prob. 9.59EPCh. 9 - What are the two alternating structure units...Ch. 9 - Draw a complete condensed structural...Ch. 9 - Draw a complete condensed structural...Ch. 9 - With the help of Table 20-1, identify the amino...Ch. 9 - Prob. 9.64EPCh. 9 - Prob. 9.65EPCh. 9 - With the help of Table 20-1, assign an IUPAC name...Ch. 9 - Prob. 9.67EPCh. 9 - Draw condensed structural formulas for the...Ch. 9 - Prob. 9.69EPCh. 9 - For the tripeptide SerArgIle which amino acid...Ch. 9 - Prob. 9.71EPCh. 9 - Consider the tripeptide leucylvalyltryptophan. a....Ch. 9 - Explain why the notations SerCys and CysSer...Ch. 9 - Explain why the notations AlaGlyValAla and...Ch. 9 - Prob. 9.75EPCh. 9 - There are a total of six different amino acid...Ch. 9 - Compare the structures of the protein hormones...Ch. 9 - Compare the protein hormones oxytocin and...Ch. 9 - Prob. 9.79EPCh. 9 - Compare the structures of the peptide...Ch. 9 - Prob. 9.81EPCh. 9 - Prob. 9.82EPCh. 9 - Prob. 9.83EPCh. 9 - Prob. 9.84EPCh. 9 - Indicate whether each of the following statements...Ch. 9 - Indicate whether each of the following statements...Ch. 9 - Prob. 9.87EPCh. 9 - Two proteins with the same amino acid composition...Ch. 9 - Prob. 9.89EPCh. 9 - Prob. 9.90EPCh. 9 - Prob. 9.91EPCh. 9 - Prob. 9.92EPCh. 9 - Prob. 9.93EPCh. 9 - Prob. 9.94EPCh. 9 - Prob. 9.95EPCh. 9 - Prob. 9.96EPCh. 9 - Prob. 9.97EPCh. 9 - Prob. 9.98EPCh. 9 - Prob. 9.99EPCh. 9 - Why is the phrase unstructured segment of a...Ch. 9 - State the four types of attractive forces that...Ch. 9 - Prob. 9.102EPCh. 9 - Prob. 9.103EPCh. 9 - Prob. 9.104EPCh. 9 - Prob. 9.105EPCh. 9 - Prob. 9.106EPCh. 9 - Indicate whether each of the following statements...Ch. 9 - Prob. 9.108EPCh. 9 - Prob. 9.109EPCh. 9 - Prob. 9.110EPCh. 9 - Prob. 9.111EPCh. 9 - Quaternary protein structure is more easily...Ch. 9 - Prob. 9.113EPCh. 9 - Prob. 9.114EPCh. 9 - Prob. 9.115EPCh. 9 - Prob. 9.116EPCh. 9 - Prob. 9.117EPCh. 9 - How many different di- and tripeptides could be...Ch. 9 - Identify the primary structure of a hexapeptide...Ch. 9 - Identify the primary structure of a hexapeptide...Ch. 9 - Draw structural formulas for the products obtained...Ch. 9 - Prob. 9.122EPCh. 9 - Prob. 9.123EPCh. 9 - Why is complete hydrolysis of a protein not also...Ch. 9 - In what way is the protein in a cooked egg the...Ch. 9 - Why is cooked protein more easily digested than...Ch. 9 - Prob. 9.127EPCh. 9 - Indicate whether or not each of the following...Ch. 9 - Prob. 9.129EPCh. 9 - Prob. 9.130EPCh. 9 - Prob. 9.131EPCh. 9 - What is the major biochemical function of each of...Ch. 9 - Using the list in Section 20-17, characterize each...Ch. 9 - Prob. 9.134EPCh. 9 - Prob. 9.135EPCh. 9 - Prob. 9.136EPCh. 9 - What two nonstandard amino acids are present in...Ch. 9 - Prob. 9.138EPCh. 9 - Prob. 9.139EPCh. 9 - Prob. 9.140EPCh. 9 - Prob. 9.141EPCh. 9 - Prob. 9.142EPCh. 9 - Prob. 9.143EPCh. 9 - Describe the process by which blood...Ch. 9 - Prob. 9.145EPCh. 9 - Prob. 9.146EPCh. 9 - Prob. 9.147EPCh. 9 - Prob. 9.148EPCh. 9 - Prob. 9.149EPCh. 9 - As the lipid content of a plasma lipoprotein...Ch. 9 - Prob. 9.151EPCh. 9 - Prob. 9.152EP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For the condensation reaction between Alamine and histamine, please help me write the amididation reaction mechanism. Then write the three letter code for the product of the reaction, then write the one letter code for the product of the reaction. arrow_forwardHow to draw the reaction mechasnism belowarrow_forwardName the following molecules with IUpacarrow_forward
- What is the molecular orbital for cyclopropenyl anion and is it aromatic, antiaromatic or nonaromatic?arrow_forwardUsing the chart describe the change from cystine to tyrosine and its impact on the protein. Using the chart describe the change from histidine to aspartic acid and its impact on the protein.arrow_forwardHow to get the predicted product of this reaction belowarrow_forward
- Please help me fill out the chart then using the chart describe the change from cystine to tyrosine and its impact on the protein. Then using the chart describe the change from histidine to aspartic acid.arrow_forwardWrite the Esterification reaction mechanism for acetic acid, and one propanol to make propanol ethanoate (molecule that gives peas its odor in flavor)arrow_forwardProvide solutionsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

World of Chemistry
Chemistry
ISBN:9780618562763
Author:Steven S. Zumdahl
Publisher:Houghton Mifflin College Div
Biomolecules - Protein - Amino acids; Author: Tutorials Point (India) Ltd.;https://www.youtube.com/watch?v=ySNVPDHJ0ek;License: Standard YouTube License, CC-BY