
ORGANIC CHEMISTRY BOOK& SG/SM
6th Edition
ISBN: 9781264094493
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 9, Problem 56P
Draw the products formed when each ether is treated with two equivalents of
a. 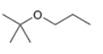 b.
b. 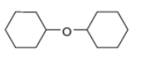 c.
c. 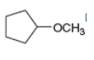
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
What is the molarity of a 0.393 m glucose solution if its density is 1.16 g/mL? MM glucose 180.2 g/mol
The rate constant for the decay of a radioactive element is 2.28 × 10⁻³ day⁻¹. What is the half-life of this element in days?
Handwritten please
Chapter 9 Solutions
ORGANIC CHEMISTRY BOOK& SG/SM
Ch. 9.1 - Problem 9.1 Label each ether and alcohol in...Ch. 9.3 - Give the IUPAC name for each compound.Ch. 9.3 - Problem 9.3 Give the structure corresponding to...Ch. 9.3 - Name each of the following ethers.Ch. 9.3 - Name each epoxide.
a. (two ways) b. c. (two...Ch. 9.6 - Problem 9.8 Draw the organic product of each...Ch. 9.6 - Prob. 9PCh. 9.6 - Problem 9.10 Draw the products of each reaction.
...Ch. 9.8 - Problem 9.11 Draw the products formed when each...Ch. 9.8 - Prob. 12P
Ch. 9.11 - Problem 9.18 Draw the products of each reaction,...Ch. 9.11 - Problem 9.19 What is the major product formed...Ch. 9.12 - Prob. 19PCh. 9.12 - Prob. 20PCh. 9.12 - Problem 9.22 Draw the organic products formed in...Ch. 9.12 - Problem 9.23 Draw two steps to convert into each...Ch. 9.13 - Prob. 23PCh. 9.13 - Problem 9.25 Draw the products of each reaction,...Ch. 9.13 - Draw the products formed when (S)-butan-2-ol is...Ch. 9.13 - Draw the product formed when (CH3)2CHOH is treated...Ch. 9.14 - What alkyl halides are formed when each ether is...Ch. 9.14 - Explain why the treatment of anisole with HBr...Ch. 9.15 - Name each thiol.
a. b.
Ch. 9.15 - Draw the product of each reaction. ac b.d.Ch. 9.15 - Give the IUPAC name for each sulfide.
a. b.
Ch. 9.15 - Draw the product of each reaction.
a. b.
Ch. 9.16 - Prob. 33PCh. 9.16 - The cis and trans isomers of 2, 3-dimethyloxirane...Ch. 9.16 - Problem 9.36 Draw the product of each...Ch. 9 - 9.37 Name each compound depicted in the...Ch. 9 - Answer each question using the ball-and-stick...Ch. 9 - Prob. 38PCh. 9 - 9.40 Give IUPAC name for each...Ch. 9 - Prob. 40PCh. 9 - Prob. 41PCh. 9 - 9.46 What alkenes are formed when each alcohol is...Ch. 9 - Prob. 46PCh. 9 - Prob. 51PCh. 9 - 9.57 Draw a stepwise, detailed mechanism for the...Ch. 9 - Prob. 53PCh. 9 - 9.59 Draw two different routes to each of the...Ch. 9 - Prob. 55PCh. 9 -
9.61 Draw the products formed when each ether is...Ch. 9 - 9.62 Draw a stepwise mechanism for each...Ch. 9 - Draw a stepwise, detailed mechanism for the...Ch. 9 - Prob. 59PCh. 9 - Draw the products of each reaction. a.c. b.d.Ch. 9 - Prob. 64PCh. 9 - Prob. 75P
Additional Science Textbook Solutions
Find more solutions based on key concepts
1. Rub your hands together vigorously. What happens? Discuss the energy transfers and transformations that take...
College Physics: A Strategic Approach (3rd Edition)
Sea turtles have disappeared from many regions, and one way of trying to save them is to reintroduce them to ar...
MARINE BIOLOGY
Describe the evolution of mammals, tracing their synapsid lineage from early amniote ancestors to true mammals....
Loose Leaf For Integrated Principles Of Zoology
Give the IUPAC name for each compound.
Organic Chemistry
Separate the list P,F,V,,T,a,m,L,t, and V into intensive properties, extensive properties, and nonproperties.
Fundamentals Of Thermodynamics
An obese 55-year-old woman consults her physician about minor chest pains during exercise. Explain the physicia...
Biology: Life on Earth with Physiology (11th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Choose the best reagents to complete the following reaction. i H A B 1. CH3CH2Na 2. H3O+ 1. CH3CH2MgBr 2. H3O+ 1. CH3MgBr Q C 2. H3O+ 1. H3O+ D 2. CH3MgBr 00 OH Q E CH³MgBrarrow_forwardThe kinetics of a gas phase reaction of the form A → Products results in a rate constant of 0.00781 M/min. For this reaction, the initial concentration of A is 0.501 M. What is the half-life for this reaction?arrow_forwardChoose the best reagents to complete the following reaction. 1. PhNa A 2. H3O+ 1. PhCH2MgBr B 2. H3O+ хё 1. PhMgBr C 2. H3O+ 00 HO Q E D 1. H3O+ 2. PhMgBr PhMgBrarrow_forward
- Please answer all of the questions and provide detailed explanations and include a drawing to show the different signals on the molecule and include which ones should be highlighted.arrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Incorrect, 1 attempt remaining 1. LiAlH4 2. H3O+ Q OH ☑ Select to Drawarrow_forwardHow should I graph my data for the Absorbance of Pb and Fe for each mushroom? I want to compare the results to the known standard curve. Software: Excel Spreadsheets Link: https://mnscu-my.sharepoint.com/:x:/g/personal/vi2163ss_go_minnstate_edu/Eb2PfHdfEtBJiWh0ipHZ_kkBW4idWWwvpLPPtqoq2WkgbQ?rtime=HxrF0_tR3Ugarrow_forward
- Provide the proper IUPAC name only for the following compound. Dashes, commas, and spaces must be used correctly, but do not use italics in Canvas.arrow_forwardThe kinetics of a gas phase reaction of the form A → Products results in a rate constant of 0.00781 M/min. For this reaction, the initial concentration of A is 0.501 M. How many minutes will it take for the concentration of A to reach 0.144 Marrow_forwardWhat is the rate for the second order reaction A → Products when [A] = 0.256 M? (k = 0.761 M⁻¹s⁻¹)arrow_forward
- For reaction N2(g) + O2(g) --> 2NO(g) Write the rate of the reaction in terms of change of NO.arrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forwardThe reaction of 2-oxacyclopentanone with hydrochloric acid in water (i.e., "excess") produces which of the following carboxylic acids?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY