
ORGANIC CHEMISTRY BOOK& SG/SM
6th Edition
ISBN: 9781264094493
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 9.14, Problem 27P
What
a.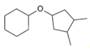 b.
b.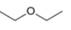 c.
c.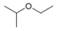
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
What is the product of the reaction of XeF4 with H2O?
Group of answer choices
H2XeF2
H2XeF4
XeO3
H2XeO
While noble gas exerts the strongest London (dispersion) forces on neighboring atoms?
Group of answer choices
Xe
Ar
Kr
Ne
Which of the following elements is corrosive to your skin due to that element breaking down C=C bonds?
Group of answer choices
fluorine
iodine
bromine
chlorine
Chapter 9 Solutions
ORGANIC CHEMISTRY BOOK& SG/SM
Ch. 9.1 - Problem 9.1 Label each ether and alcohol in...Ch. 9.3 - Give the IUPAC name for each compound.Ch. 9.3 - Problem 9.3 Give the structure corresponding to...Ch. 9.3 - Name each of the following ethers.Ch. 9.3 - Name each epoxide.
a. (two ways) b. c. (two...Ch. 9.6 - Problem 9.8 Draw the organic product of each...Ch. 9.6 - Prob. 9PCh. 9.6 - Problem 9.10 Draw the products of each reaction.
...Ch. 9.8 - Problem 9.11 Draw the products formed when each...Ch. 9.8 - Prob. 12P
Ch. 9.11 - Problem 9.18 Draw the products of each reaction,...Ch. 9.11 - Problem 9.19 What is the major product formed...Ch. 9.12 - Prob. 19PCh. 9.12 - Prob. 20PCh. 9.12 - Problem 9.22 Draw the organic products formed in...Ch. 9.12 - Problem 9.23 Draw two steps to convert into each...Ch. 9.13 - Prob. 23PCh. 9.13 - Problem 9.25 Draw the products of each reaction,...Ch. 9.13 - Draw the products formed when (S)-butan-2-ol is...Ch. 9.13 - Draw the product formed when (CH3)2CHOH is treated...Ch. 9.14 - What alkyl halides are formed when each ether is...Ch. 9.14 - Explain why the treatment of anisole with HBr...Ch. 9.15 - Name each thiol.
a. b.
Ch. 9.15 - Draw the product of each reaction. ac b.d.Ch. 9.15 - Give the IUPAC name for each sulfide.
a. b.
Ch. 9.15 - Draw the product of each reaction.
a. b.
Ch. 9.16 - Prob. 33PCh. 9.16 - The cis and trans isomers of 2, 3-dimethyloxirane...Ch. 9.16 - Problem 9.36 Draw the product of each...Ch. 9 - 9.37 Name each compound depicted in the...Ch. 9 - Answer each question using the ball-and-stick...Ch. 9 - Prob. 38PCh. 9 - 9.40 Give IUPAC name for each...Ch. 9 - Prob. 40PCh. 9 - Prob. 41PCh. 9 - 9.46 What alkenes are formed when each alcohol is...Ch. 9 - Prob. 46PCh. 9 - Prob. 51PCh. 9 - 9.57 Draw a stepwise, detailed mechanism for the...Ch. 9 - Prob. 53PCh. 9 - 9.59 Draw two different routes to each of the...Ch. 9 - Prob. 55PCh. 9 -
9.61 Draw the products formed when each ether is...Ch. 9 - 9.62 Draw a stepwise mechanism for each...Ch. 9 - Draw a stepwise, detailed mechanism for the...Ch. 9 - Prob. 59PCh. 9 - Draw the products of each reaction. a.c. b.d.Ch. 9 - Prob. 64PCh. 9 - Prob. 75P
Additional Science Textbook Solutions
Find more solutions based on key concepts
Gregor Mendel never saw a gene, yet he concluded that some inherited factors were responsible for the patterns ...
Campbell Essential Biology (7th Edition)
Give the IUPAC name for each compound.
Organic Chemistry
An obese 55-year-old woman consults her physician about minor chest pains during exercise. Explain the physicia...
Biology: Life on Earth with Physiology (11th Edition)
What were the major microbiological interests of Martinus Beijerinck and Sergei Winogradsky? It can be said tha...
Brock Biology of Microorganisms (15th Edition)
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
6. How can you use the features found in each chapter?
Human Anatomy & Physiology (2nd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What the best source of sulfide to use on a small scale in the lab? Group of answer choices thiourea H2S NaHS Na2Sarrow_forwardWhich of the following statements about sulfur is FALSE? Group of answer choices H2S is the product of an oxygen-depleted ecosystem. In the acid mine drainage reaction, FeS2 is a product. One allotrope of sulfur has the formula S20. In the environment, bacterial oxidation can convert S2− to elemental S or SO42−.arrow_forwardOf the following choices, which is the best reason that most materials DON'T spontaneously combust even though our atmosphere is about 21% oxygen? Group of answer choices The reduction of O2 in the gas phase (O2 + e− → O2−) is spontaneous. The reduction of O2 in acid solution (O2 + H+ + e− → HO2(aq)) is spontaneous. O2 is not a reactant in combustion. The O2 bond dissociation energy is 494 kJ/mol, leading to a high activation energy for combustion.arrow_forward
- please answer in the scope of the SCH4U course, I am having a hard time understanding, may you show all steps please and thank you! can you also put the final answers in the table so its understandablearrow_forwardPlan the synthesis of the following compound using the starting material provided and any other reagents needed as long as carbon based reagents have 3 carbons or less. Either the retrosynthesis or the forward synthesis (mechanisms are not required but will be graded if provided) will be accepted if all necessary reagents and intermediates are shown (solvents and temperature requirements are not needed unless specifically involved in the reaction, i.e. DMSO in the Swem oxidation or heat in the KMnO4 oxidation). There may be more than one correct answer, and chemically correct steps will be accepted. Extra points will be given if correct names are provided. The points earned here will be applied to your lowest exam score! H Harrow_forwardDraw the mechanism to make the alcohol 1-hexanol. Please use arrows.arrow_forward
- Answer the followings: 1-What is the difference(s) between DNA and RNA: a- Structure: b- Function: c- Types: 2-What is the meaning of: a- Replication b- Transcription c- Translation 3- Show the base pair connection (hydrogen bond) in DNA and RNAarrow_forwardWhy does the anhydride react with the OH on the benzene rather than the OH on the carboxy group?arrow_forwardAnswer the followings: 1- What is the IP for a amino acid? Give example. 2- What are the types of amino acids? 3- What are the structures of protein? 4- The N-Terminal analysis by the Edman method shows saralasin contains sarcosine at the N- terminus. Partial hydrolysis of saralasin with dilute hydrochloric acid yields the following fragments: Tyr-Val-His Sar-Arg-Val His-Pro-Ala Val-Tyr-Val Arg-Val-Tyr What is the structure of saralasin? 5. MATCH a term from the list below to each definition. Place the letter of the term in the blank to the left of the definition. a. Ligases b. Fibrous proteins c. Conjugated protein d. Hydrolases a. b. C. e. Simple protein f. Globular proteins g. Lyases h. Transferases Proteins that are tough and insoluble in water. Enzymes that catalyze the breaking away of a small molecule such as from a substrate. Enzymes that catalyze the bonding together of two substrates.arrow_forward
- Answer the followings (Four): 1-What is the difference(s) between FOUR: a. Glyceride and phosphoglyceride. b. Wax and fat. c. Soap and fatty acid. d. HDL and LDL cholesterol e. Phospho lipids and sphingosine. 2-What are the types of lipids? 3-What are the main lipid components of membrane structures? 4-How could lipids play important rules as signaling molecules and building units? 5. The Structure variety of Lipids makes them to play significant rules in our body. Conclude briefly on this statement.arrow_forwardHO IV но. = HO но. HO. HO но. зад надо What is the product of the following reaction?arrow_forwardDraw the mechanism to make the alcohol 2-hexanol.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY