
a)
Interpretation:
The difference in the boiling points of the given set of compounds has to be rationalized.
Concept Introduction:
- Boiling point of any compound, depends upon its strength of Intramolecular force and Intermolecular force present in it.
- Intramolecular force refers to type of bonding between the atoms.
- Intermolecular forces are the forces that bind the molecules together to attribute to a stability of a compound.
- If the strength of intermolecular forces is high, boiling point will be high and if it is low, boiling point will be low.
- They are collectively known as “Interparticle forces”. The classification can be summarized as follows –
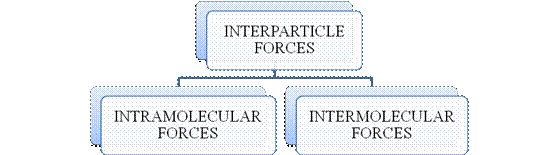
Figure 1
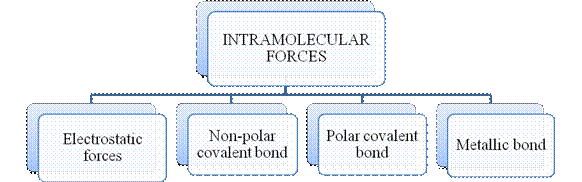
Figure 2
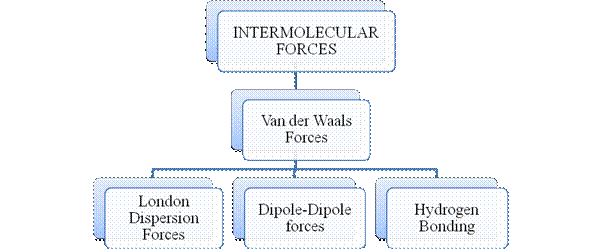
Figure 3
The type of bonding between atoms or ions is Intramolecular force. The intramolecular force in ionic compounds is electrostatic force of attraction between the ions of opposite charges. Usually ionic compounds are solids with high melting points. Covalent bonds are of two types, that is polar covalent bond and non-polar covalent bond. Covalent compounds are found as solids and liquids with moderate melting and boiling point. Metallic bond is formed between the metal atoms of an element.
Intermolecular forces are Van der Waals forces. They are weak and are of three types - London dispersion forces, dipole-dipole forces and hydrogen bonding. Hydrogen bonding is formed in polar covalent compounds containing hydrogen and other high electronegativity like fluorine, oxygen or nitrogen. These atoms in a molecule partially bond to hydrogen of the other same molecule or within a molecule. It is relatively the strongest one. Hydrogen bonded compounds are usually liquids. They exhibit high boiling point.
London dispersion forces exist in all types of molecules. This is the force responsible for the condensation of non-polar compounds into liquids or solids under low temperature.
Dipole-dipole forces exist in polar covalent compounds. Hydrogen bonding exists in polar covalent compounds containing Fluorine, Oxygen or Nitrogen directly bonded to Hydrogen.
b)
Interpretation:
The difference in the boiling points of the given set of compounds has to be rationalized.
Concept Introduction:
- Boiling point of any compound, depends upon its strength of Intramolecular force and Intermolecular force present in it.
- Intramolecular force refers to type of bonding between the atoms.
- Intermolecular forces are the forces that bind the molecules together to attribute to a stability of a compound.
- If the strength of intermolecular forces is high, boiling point will be high and if it is low, boiling point will be low.
- They are collectively known as “Interparticle forces”. The classification can be summarized as follows –
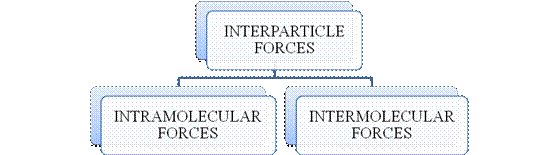
Figure 1
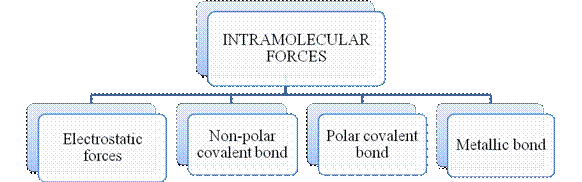
Figure 2
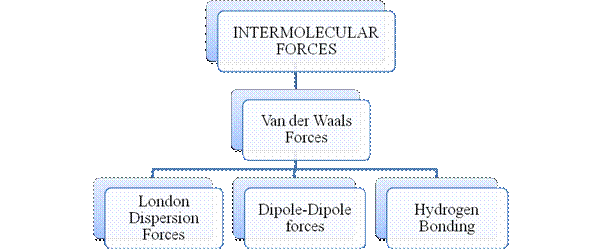
Figure 3
The type of bonding between atoms or ions is Intramolecular force. The intramolecular force in ionic compounds is electrostatic force of attraction between the ions of opposite charges. Usually ionic compounds are solids with high melting points. Covalent bonds are of two types, that is polar covalent bond and non-polar covalent bond. Covalent compounds are found as solids and liquids with moderate melting and boiling point. Metallic bond is formed between the metal atoms of an element.
Intermolecular forces are Van der Waals forces. They are weak and are of three types - London dispersion forces, dipole-dipole forces and hydrogen bonding. Hydrogen bonding is formed in polar covalent compounds containing hydrogen and other high electronegativity like fluorine, oxygen or nitrogen. These atoms in a molecule partially bond to hydrogen of the other same molecule or within a molecule. It is relatively the strongest one. Hydrogen bonded compounds are usually liquids. They exhibit high boiling point.
London dispersion forces exist in all types of molecules. This is the force responsible for the condensation of non-polar compounds into liquids or solids under low temperature.
Dipole-dipole forces exist in polar covalent compounds. Hydrogen bonding exists in polar covalent compounds containing Fluorine, Oxygen or Nitrogen directly bonded to Hydrogen.
c)
Interpretation:
The difference in the boiling points of the given set of compounds has to be rationalized.
Concept Introduction:
- Boiling point of any compound, depends upon its strength of Intramolecular force and Intermolecular force present in it.
- Intramolecular force refers to type of bonding between the atoms.
- Intermolecular forces are the forces that bind the molecules together to attribute to a stability of a compound.
- If the strength of intermolecular forces is high, boiling point will be high and if it is low, boiling point will be low.
- They are collectively known as “Interparticle forces”. The classification can be summarized as follows –
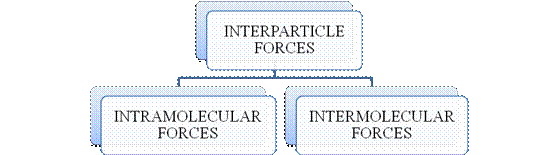
Figure 1

Figure 2
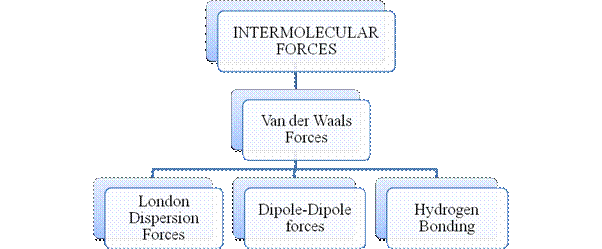
Figure 3
The type of bonding between atoms or ions is Intramolecular force. The intramolecular force in ionic compounds is electrostatic force of attraction between the ions of opposite charges. Usually ionic compounds are solids with high melting points. Covalent bonds are of two types, that is polar covalent bond and non-polar covalent bond. Covalent compounds are found as solids and liquids with moderate melting and boiling point. Metallic bond is formed between the metal atoms of an element.
Intermolecular forces are Van der Waals forces. They are weak and are of three types - London dispersion forces, dipole-dipole forces and hydrogen bonding. Hydrogen bonding is formed in polar covalent compounds containing hydrogen and other high electronegativity like fluorine, oxygen or nitrogen. These atoms in a molecule partially bond to hydrogen of the other same molecule or within a molecule. It is relatively the strongest one. Hydrogen bonded compounds are usually liquids. They exhibit high boiling point.
London dispersion forces exist in all types of molecules. This is the force responsible for the condensation of non-polar compounds into liquids or solids under low temperature.
Dipole-dipole forces exist in polar covalent compounds. Hydrogen bonding exists in polar covalent compounds containing Fluorine, Oxygen or Nitrogen directly bonded to Hydrogen.
d)
Interpretation:
The difference in the boiling points of the given set of compounds has to be rationalized.
Concept Introduction:
- Boiling point of any compound, depends upon its strength of Intramolecular force and Intermolecular force present in it.
- Intramolecular force refers to type of bonding between the atoms.
- Intermolecular forces are the forces that bind the molecules together to attribute to a stability of a compound.
- If the strength of intermolecular forces is high, boiling point will be high and if it is low, boiling point will be low.
- They are collectively known as “Interparticle forces”. The classification can be summarized as follows –
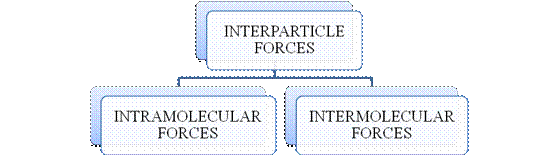
Figure 1

Figure 2

Figure 3
The type of bonding between atoms or ions is Intramolecular force. The intramolecular force in ionic compounds is electrostatic force of attraction between the ions of opposite charges. Usually ionic compounds are solids with high melting points. Covalent bonds are of two types, that is polar covalent bond and non-polar covalent bond. Covalent compounds are found as solids and liquids with moderate melting and boiling point. Metallic bond is formed between the metal atoms of an element.
Intermolecular forces are Van der Waals forces. They are weak and are of three types - London dispersion forces, dipole-dipole forces and hydrogen bonding. Hydrogen bonding is formed in polar covalent compounds containing hydrogen and other high electronegativity like fluorine, oxygen or nitrogen. These atoms in a molecule partially bond to hydrogen of the other same molecule or within a molecule. It is relatively the strongest one. Hydrogen bonded compounds are usually liquids. They exhibit high boiling point.
London dispersion forces exist in all types of molecules. This is the force responsible for the condensation of non-polar compounds into liquids or solids under low temperature.
Dipole-dipole forces exist in polar covalent compounds. Hydrogen bonding exists in polar covalent compounds containing Fluorine, Oxygen or Nitrogen directly bonded to Hydrogen.
Want to see the full answer?
Check out a sample textbook solution
Chapter 9 Solutions
Bundle: Chemistry: An Atoms First Approach, Loose-leaf Version, 2nd + OWLv2 with Student Solutions Manual, 4 terms (24 months) Printed Access Card
- I have a 2 mil plastic film that degrades after 22 days at 88C and at 61C takes 153 days. What is the failure at 47C in days.arrow_forwardIf a 5 film plastic film degraded in 30 days at 35C and the same film degraded in 10 days at 55 C and 2 days at 65C what would the predicted life time be at 22C for the same film?arrow_forwardno Ai walkthroughsarrow_forward
- I have a aqueous solution (175 ml) of iridium trichloride containing 8,750 ppm Iridium by ICP OES analysis. What is the percent concentration of Iridium trichloride in aquous solution and provide the concentration in moles per liter, percentage by weight.arrow_forwardno Ai walkthroughsarrow_forwardno Ai walkthroughsarrow_forward
- no Ai walkthroughs (product in picture is wrong btw don't submit the same thing)arrow_forwardno Ai walkthroughsarrow_forward136 PRACTICAL SPECTROSCOPY Compound 78 is a high-boiling liquid (boiling point 189° C) that contains halogen, but will not react with alkoxides to yield an halogen. ether. The Mass, IR, and 'H NMR spectra, along with 13C NMR data, are given below. Elemental Analysis: C, 35.32; H, 2.47; contains BC Spectral Data: doublet, 137.4 ppm; doublet, 130.1 ppm; doublet, 127.4 ppm; singlet, 97.3 ppm Absorbance Mass Spectrum Intensity 77 77 204 M + 128 40 60 80 100 120 140 160 180 m/e 200 220 280 240 260 300 Infrared Spectrum Wave Number, cm -1 4000 3000 2500 2000 1500 1300 1200 1100 1000 900 800 700 3 6 7 8 9 10 12 13 15 Wavelength, microns 'H NMR wwwww 5 Structure: www ppm, & ©2000 Brooks/Cole Publishing Com-arrow_forward
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning





